Test strip for detecting HIV (Human Immunodeficiency Virus) antibody in urea, detection cup and preparation method of test strip
A technology of test strips and antibodies, applied in the field of medicine, can solve the problems of high false positives in saliva, only qualitative detection, low sensitivity, etc., and achieve the effects of ensuring authenticity, reliability, convenient transportation, and wide cycle range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
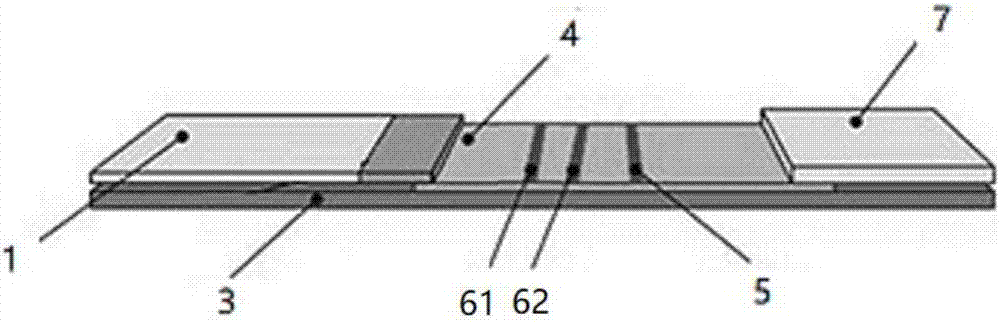
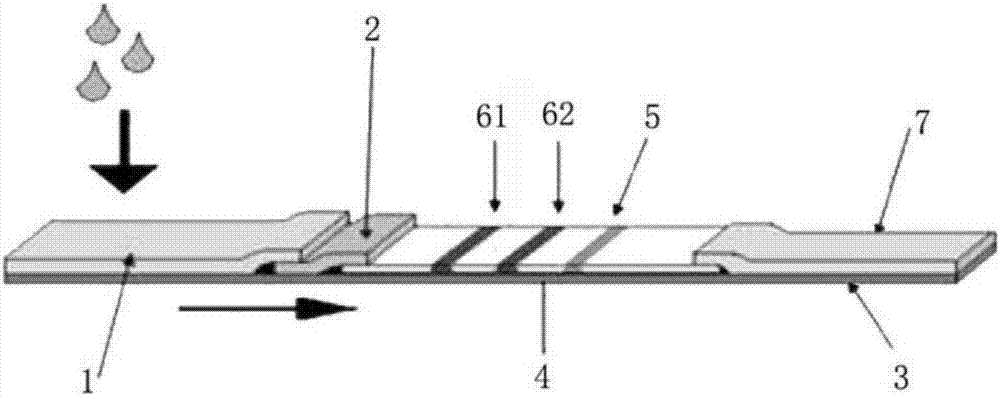
[0040] see figure 1 , a test strip for detecting HIV antibodies in urine according to the present embodiment, the test strip includes a base plate 3, and a sample pad 1 and a nitrocellulose membrane 4 arranged on the base plate 3, the sample pad 1 adopts The sample pad coating liquid is processed, and the two ends of the nitrocellulose membrane 4 are overlapped with the sample pad 1 and the absorbent paper 7 respectively, and the colloidal gold-labeled mouse anti-human IgG antibody is sprayed on the sample pad 1, and the colloidal gold Labeled gp160 antigen and colloidal gold labeled gp36 antigen, the nitrocellulose membrane 4 is provided with a first detection line 61 coated with HIV recombinant antigen gp41 and HIV recombinant antigen gp160, and a second detection line 62 coated with HIV recombinant antigen gp36 , and the control line 5 coated with goat anti-mouse antibody.
[0041] Wherein, the sample pad treatment liquid comprises Tris-HCl buffer solution, HPMC, PAA, rabb...
Embodiment 2
[0054] The structure and preparation method of the test strip for detecting HIV antibodies in urine of the present embodiment are all the same as in Example 1, except that the concentration of the colloidal gold-labeled gp160 antigen is 1.5 μg / ml; the colloidal gold-labeled mouse The concentration of the anti-human IgG antibody was 4.5 μg / ml; the concentration of the colloidal gold-labeled gp36 antigen was 4.5 μg / ml. The amount of the colloidal gold labeled raw material is 2ul / cm; the coating concentration of the HIV recombinant antigen gp41 is 0.5mg / ml, the coating concentration of the HIV recombinant antigen gp160 is 0.2mg / ml, and the HIV recombinant antigen The coating concentration of gp36 is 0.8mg / ml, and the dosage of the HIV recombinant antigen is 0.15ul / mm. The concentration of the goat anti-mouse antibody is 0.8mg / ml, and the dosage of the goat anti-mouse antibody is 0.2ul / mm.
[0055] The sample pad treatment solution in this embodiment includes Tris-HCl buffer solu...
Embodiment 3
[0058] The structure and preparation method of the test strip for detecting HIV antibodies in urine of this embodiment are all the same as in Example 1, except that the concentration of the colloidal gold-labeled gp160 antigen is 3.5 μg / ml; the colloidal gold-labeled mouse The concentration of the anti-human IgG antibody was 6.5 μg / ml; the concentration of the colloidal gold-labeled gp36 antigen was 6.5 μg / ml. The amount of the colloidal gold labeled raw material is 3.5ul / cm; the coating concentration of the HIV recombinant antigen gp41 is 2.0mg / ml, the coating concentration of the HIV recombinant antigen gp160 is 0.8mg / ml, the HIV recombinant The coating concentration of the antigen gp36 is 0.9mg / ml, and the dosage of the HIV recombinant antigen is 0.13ul / mm. The concentration of the goat anti-mouse antibody is 0.9mg / ml, and the dosage of the goat anti-mouse antibody is 0.15ul / mm.
[0059] The sample pad treatment solution of this embodiment includes Tris-HCl buffer solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com