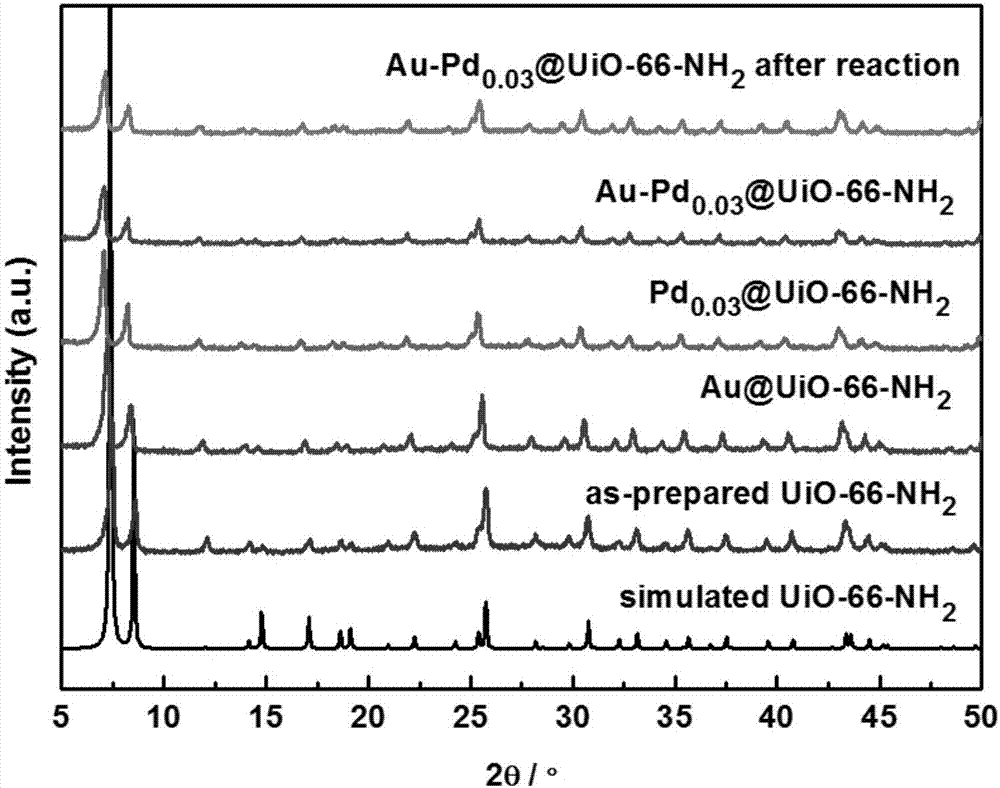
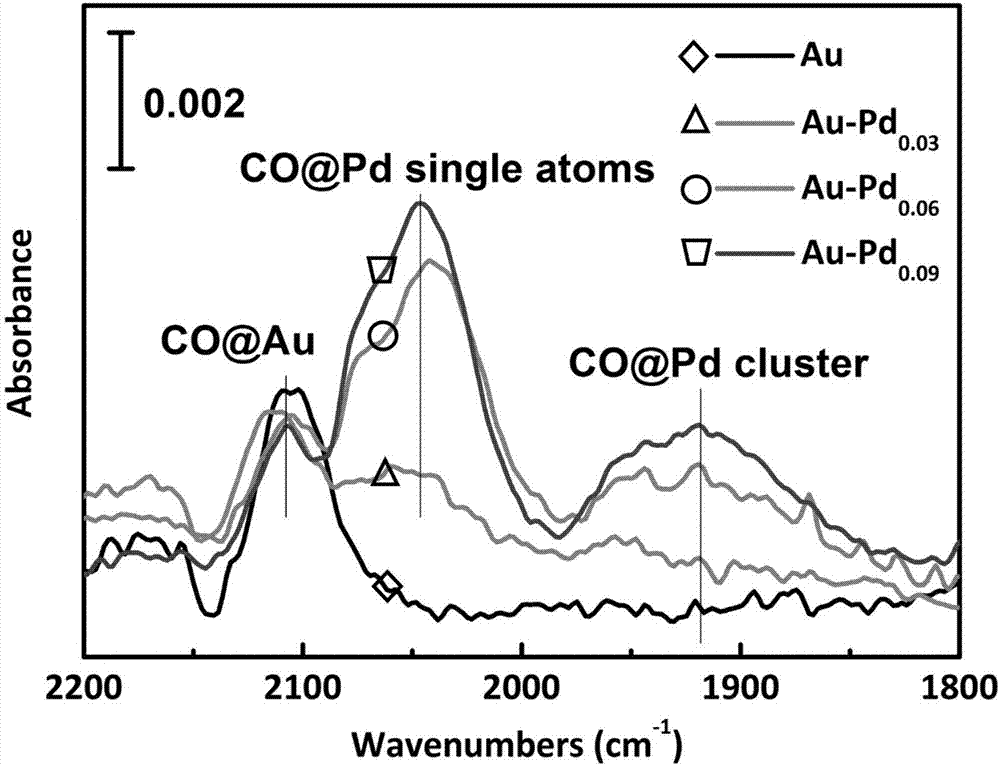
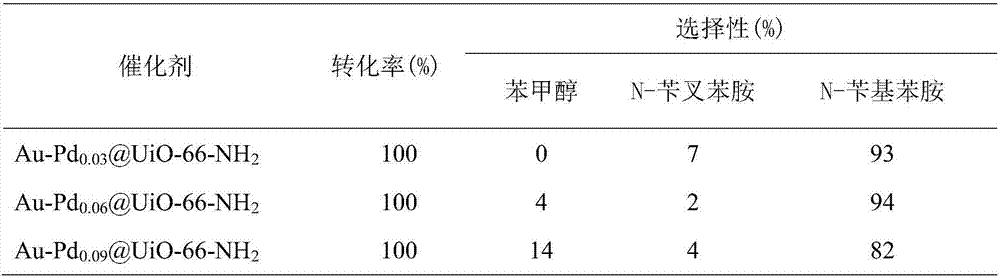
Preparation method and application of high hydrogenation selectivity Au-Pd monatomic alloy catalyst
An alloy catalyst, selective technology, applied in catalyst activation/preparation, reductive alkylation preparation, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of low hydrogenation selectivity, low hydrogen activation capacity, etc. problem, to achieve the effect of high selectivity, simple preparation method and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: UiO-66-NH 2 preparation of
[0024] Preparation of UiO-66-NH by Hydrothermal Synthesis 2 . Weigh 0.23gZrCl 4 , 0.18g of 2-aminoterephthalic acid was dissolved in a mixed solution of 50ml of N, N-dimethylformamide and 0.15ml of water, and the mixed system was stirred at room temperature for 0.5h and then transferred to a polytetrafluoro-lined autoclave , placed in an oven at 120°C for 48h. After the removed reactor was cooled to room temperature, the solid product obtained by centrifugation was washed twice with N,N-dimethylformamide, and then stirred and washed with anhydrous methanol at room temperature for 12 hours. Dry in vacuum at 80°C for 12 hours before use.
Embodiment 2
[0025] Example 2: Au-Pd 0.03 @UiO-66-NH 2 preparation of
[0026] Weigh 0.2g dry UiO-66-NH 2 Solid, dispersed in a mixed system of 10ml water and 10ml ethanol, sonicated for 0.5h until uniformly dispersed. The configuration contains 1.33×10 -3 mol / LHAuCl 4 and 0.37×10 -4 mol / LH 2 PdCl 4 15ml of the solution, under the condition of ice bath, this solution was added drop by drop to UiO-66-NH through peristaltic pump within 40min 2 in the dispersed system. After the dropwise addition was completed, the mixture was further stirred under ice bath for 4 h. Then 5 ml of newly configured 0.08 mol / L sodium borohydride solution was added dropwise to the mixed system. After further stirring for 0.5 h, the obtained Au-Pd 0.03 @UiO-66-NH 2 Then wash with water three times, and vacuum-dry at 80°C until use. Example 3: Au-Pd 0.06 @UiO-66-NH 2 preparation of
Embodiment 3
[0027] Repeat the step in embodiment 3, difference is: the H 2 PdCl 4 The concentration of the solution was changed to 0.74×10 -4 mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com