Copper-based catalyst for reverse water-gas shift reaction and preparation method therefor
A technology of copper-based catalysts and conversion reactions, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, etc., can solve problems such as copper-based catalyst instability and achieve overcoming Poor thermal stability, reduced high temperature sintering, and continuously adjustable pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
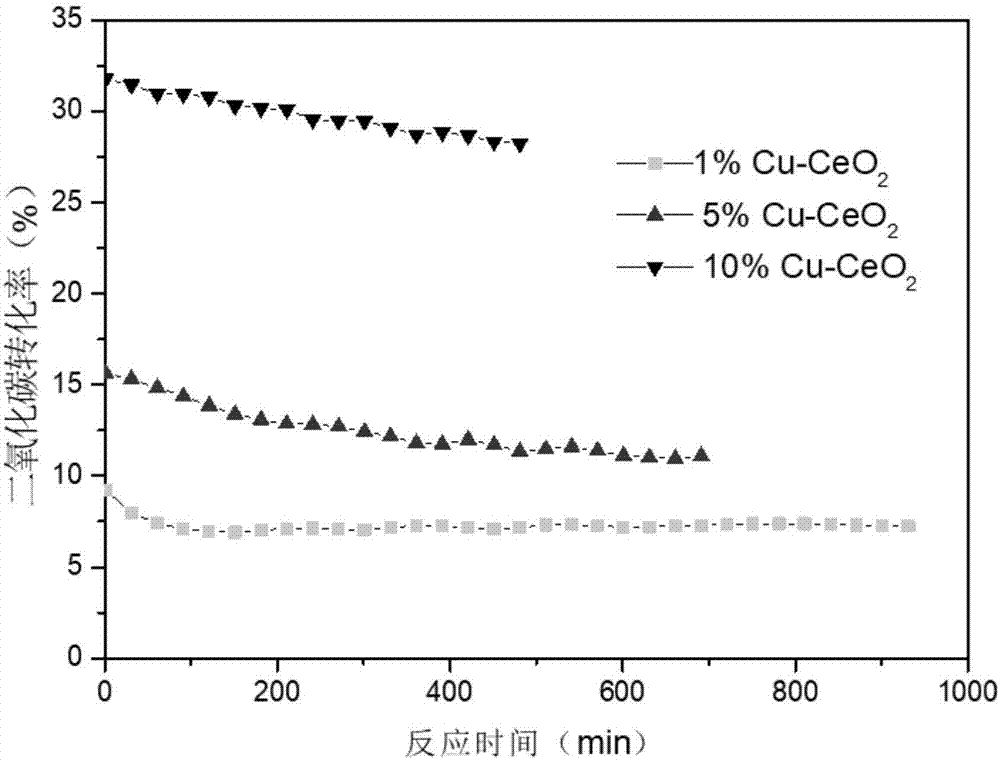
[0025] Weigh 0.038g of Cu(NO 3 ) 2 ·3H 2 O, 2.50 g of Ce (NO 3 ) 3 ·6H 2 O and 0.6g of glycine were placed in a 10ml beaker, 6.3ml of deionized water was added, and sonicated for 20min until completely dissolved. Then 1.26 ml of silica sol (neutral) was added, and ultrasonically oscillated again for 20 min. The above solution was poured into a 250 ml beaker and heated to 170°C on a hot plate until the reaction of glycine and nitrate was complete. After cooling to room temperature, the samples were placed in a muffle furnace set at a rate of 2 °C / min and calcined at room temperature from 25 °C to 600 °C for 4 h. After that, the silica sol template was removed by constant-temperature magnetic stirring at 80 °C for 4 h with 2 mol / L NaOH solution, and centrifuged and washed (water → water → ethanol → water → ethanol) for several times to obtain a solid powder, which was placed in a beaker and dried at 100 °C. Dry in the box for 6h, then cool to room temperature to obtain 1%...
Embodiment 2
[0027] Except for Cu(NO 3 ) 2 The amount of 0.19g, Ce (NO 3 ) 3 ·6H 2 The amount of O was 2.40 g, and the rest of the preparation method was exactly the same as that in Example 1 to obtain 5% Cu-CeO 2 .
Embodiment 3
[0029] Except for Cu(NO 3 ) 2 The amount of 0.38g, Ce (NO 3 ) 3 ·6H 2 The amount of O was 2.27 g, and the rest of the preparation method was exactly the same as that in Example 1 to obtain 10% Cu-CeO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com