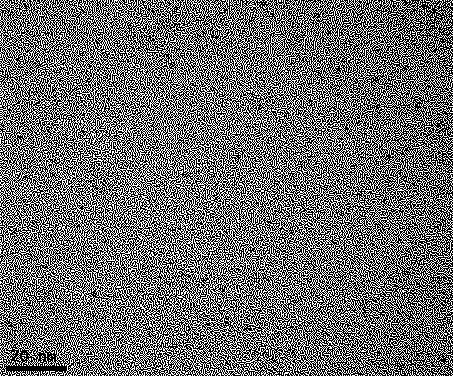Preparation method of gadolinium sulfide nanoparticles and application thereof
A nanoparticle and gadolinium sulfide technology, applied in the field of nanomaterials, can solve the problems of poor biocompatibility, large size, complex synthesis process, etc., and achieve the effects of low cost, good water dispersibility and stability, and high relaxation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention discloses a preparation method and application of gadolinium sulfide nanoparticles.
[0028] Specifically, the invention discloses a method for preparing gadolinium sulfide nanoparticles by biomimetic synthesis, comprising the following steps:
[0029] A) adding the inorganic salt solution of gadolinium to the protein molecule solution;
[0030] B) Add sodium hydroxide solution dropwise to the solution in step A) to adjust the pH value to 12;
[0031] C) Add inorganic sulfide salt solution to step B) solution, heat and stir;
[0032] D) Dialyzing and purifying the solution obtained in step C), and freeze-drying to obtain gadolinium sulfide nanoparticles.
[0033] In the method, the inorganic salt of gadolinium is selected from gadolinium nitrate, gadolinium chloride or gadolinium sulfate; the protein molecule is selected from bovine serum albumin, human serum albumin or transferrin; the inorganic sulfide is selected from sodium sulfide, potassium sulfide...
Embodiment 1
[0040] Example 1: Biomimetic synthesis of gadolinium sulfide nanoparticles
[0041] 1) Add 1ml, 100mmol / L gadolinium chloride solution to 9ml, 25mg / ml bovine serum albumin (BSA) solution, and stir;
[0042] 2) Add 1mol / L NaOH solution dropwise to the solution obtained in step 1) to adjust the pH to 12;
[0043] 3) Add 1ml, 150mmol / L sodium sulfide solution to the solution obtained in step 2), stir and react at 37°C for 4 hours,
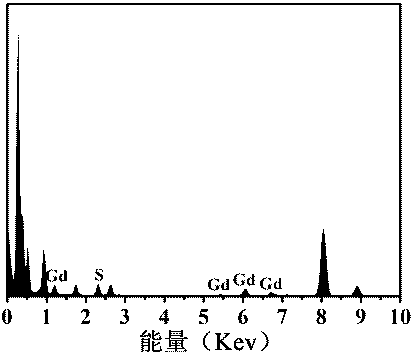
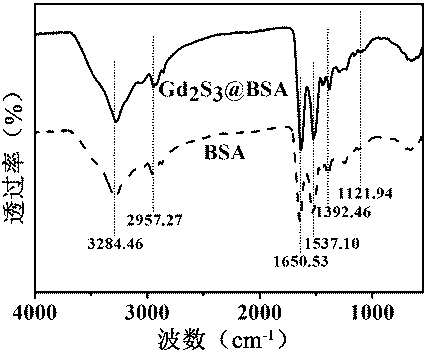
[0044] 4) The solution obtained in 3) was dialyzed for 24 hours with a dialysis bag with a molecular weight cut-off of 10,000 Da, and freeze-dried to obtain gadolinium sulfide nanoparticles. The obtained gadolinium sulfide nanoparticles were characterized by transmission electron microscope, X-ray energy spectrum and Fourier transform infrared spectrum, the results are as follows: figure 1 , 2 , 3 shown.
Embodiment 2
[0045] Embodiment 2: The relaxation rate measurement of gadolinium sulfide nanoparticles
[0046] A certain amount of gadolinium sulfide nanoparticles was weighed and dispersed in distilled water to prepare solutions with gadolinium concentrations of 2.0, 1.0, 0.5, 0.25, and 0.125 mmol / L, respectively. Use 7T nuclear magnetic resonance instrument, select RARE-T 1 +T 2 -map sequence, the parameters are set as follows: TR=200, 400, 800, 1500, 3000, 5000 ms, TE=11.00 ms, FOV=50 mm×50 mm, matrix=256 mm×256 mm, FA=180 o , slice thickness=1mm, carry out T on the prepared solution 1 weighted imaging scan, the resulting longitudinal relaxation time (T 1 ) is linearly fitted to the concentration, and the slope obtained is the relaxation rate. Its fitting curve see Figure 4 , the relaxation rate of the obtained gadolinium sulfide nanoparticles is 2.89mM -1 the s -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relaxation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com