A glucagon-like peptide-1 receptor agonist fusion protein and applications thereof
A fusion protein and receptor technology, applied in the field of fusion proteins, can solve the problems of long medication time, poor patient compliance, and inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
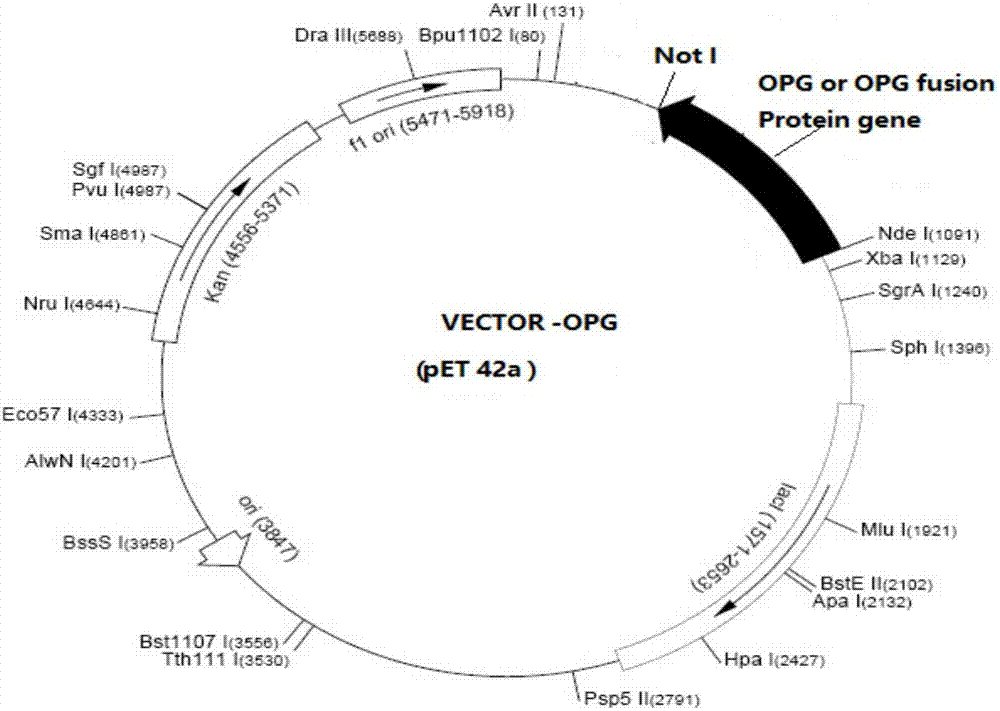
[0085] Embodiment 1: Construction of expression vector
[0086] The gene shown in SEQ ID NO.2 and the linker gene shown in SEQ ID NO.9 and the GLP-1 gene shown in SEQ ID NO.4 are connected by a fully synthetic method as shown in SEQ ID NO.13 (nomenclature OPG-A (CTB-L1-G), placed in the vector pUC57, named pUC57-OPG-A. Using SEQ ID NO.16primer F and SEQ ID NO.17primer R as the upstream and downstream primers respectively, and using the pUC57-OPG-A plasmid as the template, PCR amplification was performed, and the PCR product was digested with Nde I and Not I. Ligate with pET42a recovered by Not I double enzyme digestion, transform the ligated product into competent DH5a, pick a single clone on an LB plate containing 50ug / ml kanamycin, and extract the plasmid after correct sequencing to be the pET-OPG-A expression vector.
[0087] The gene shown in SEQ ID NO.2 and the linker gene shown in SEQ ID NO.9 and the GLP-1-a gene shown in SEQ ID NO.6 are connected by a fully synthetic m...
Embodiment 2
[0090] Embodiment 2: the expression of fusion protein
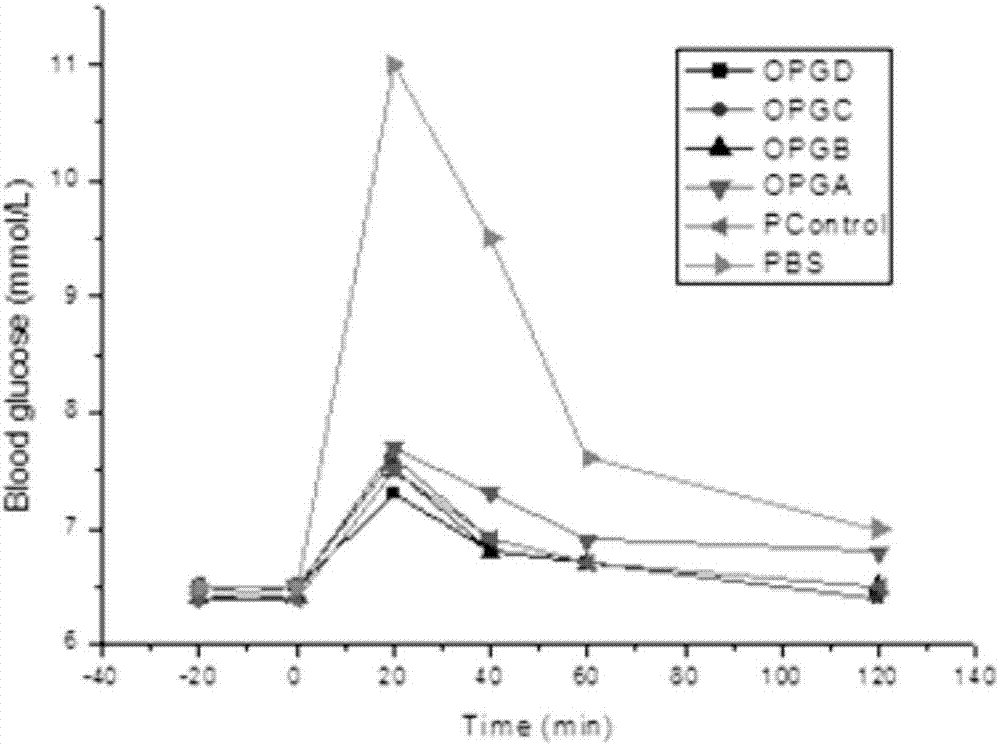
[0091] Transform the correctly constructed expression vectors pET-OPG-A, pET-OPG-B, pET-OPG-C, and pET-OPG-D into BL21(DE3) competent bacteria, respectively, in the presence of 50ug / ml kanamycin Single clones were picked on LB plates. Single clones were inoculated in LB liquid medium containing 50ug / ml kanamycin, cultured with shaking at 200rpm at 37°C, when OD 600 When 1.0 was reached, expression was induced by adding IPTG at a final concentration of 0.5 mM. Continue culturing at 30°C for 4-6 hours, collect the bacteria by centrifugation, and freeze them at -20°C.
Embodiment 3
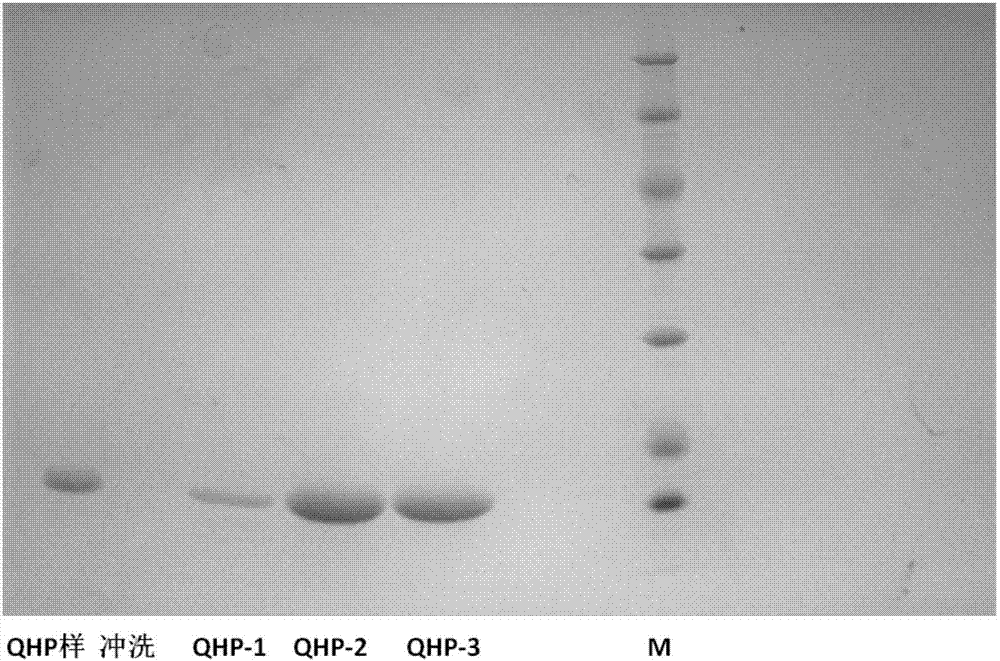
[0092] Embodiment 3: the purification of fusion protein
[0093] Dissolve the cells of OPG-A, OPG-B, and OPG-C in 20mM Tris-HCl buffer solution with pH 8.0 at a weight ratio of 1:15, where the NaCl concentration is 0.1M, crush twice under 600 bar pressure, 8000g Centrifuge to remove fragments and then filter through 0.4um and apply to QFF anion chromatography column. After equilibration, it is eluted with 20mM Tris-HCl of pH 8.0 containing 0.5M NaCl. The eluate was adjusted to 1.8M salt concentration with NaCl solid, then subjected to butyl hydrophobic chromatography, and eluted with 20 mM Tris-HCl at pH 7.5 after equilibration. The protein eluate was chromatographed on Superdex S100 gel with 10mMpH7.2PB buffer, and the main protein peak was collected, which was the target protein purification solution.
[0094] Dissolve the OPG-D bacteria in 20mM Tris-HCl buffer solution with pH 8.0 at a weight ratio of 1:15, where the NaCl concentration is 0.1M, crush twice under 600 bar pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com