Environment-friendly synthetic method of 4-ferrocenyl quinoline derivative
A ferrocene-based quinoline, derivative technology, applied in chemical instruments and methods, metallocene, organic chemistry and other directions, can solve the problems of non-conformity to green synthesis, low synthesis efficiency, heavy metal pollution, etc., and achieves low cost, green Effects of mild synthesis and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] In view of this, the present invention provides a kind of green synthetic method of 4-ferrocenyl quinoline derivative, and this synthetic method comprises:
[0022] Using an organic acid as a catalyst, ferrocenyl acetylene, aromatic aldehyde and aromatic primary amine undergo a three-component cyclization reaction in a solvent to generate 4-ferrocenyl quinoline derivatives in one step; the organic acid is p-toluenesulfonic acid , trifluoromethanesulfonic acid, trifluoroacetic acid or acetic acid, the solvent is water, ethanol or methanol;
[0023] Wherein, the structural formula of the aromatic aldehyde is as follows:
[0024] Ar-CHO
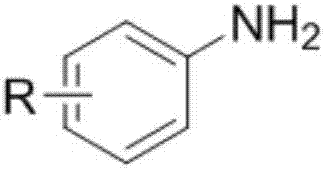
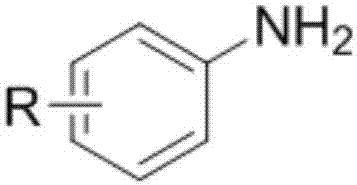
[0025] The structural formula of the aromatic primary amine is as follows:
[0026]
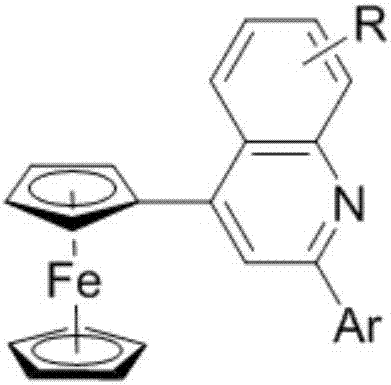
[0027] The structural formula of the 4-ferrocenyl quinoline derivative is as follows:
[0028]
[0029] In the above structural formula, R is H, CH 3 、CH 3 O, OH, NO 2 , Cl or Br, R is located at the 5, 6, 7 or 8 position of the quinoline rin...
Embodiment 1
[0046] Synthesis of 2-phenyl-4-ferrocenylquinoline
[0047] A mixture of ferroceneacetylene (0.231g, 1.1mmol), benzaldehyde (0.106g, 1.0mmol), aniline (0.093g, 1.1mmol), p-toluenesulfonic acid (0.017g, 0.1mmol) and water (1mL) , and stirred the reaction at 100° C. for 2 hours. The reaction mixture was cooled to room temperature, ethyl acetate was added, washed with saturated sodium bicarbonate solution and brine respectively, the organic phase was dried over anhydrous magnesium sulfate, and the solvent was distilled off from the filtrate under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography (silica gel: 200-300 mesh, eluent: ethyl acetate / petroleum ether=1:20, v / v, R f =0.3) to obtain a red solid with a yield of 90%, m.p.145-146°C. HR-MS: Calcd for C 25 h 19 FeN[M] + :390.0945,Found:390.0975; 1 H NMR (CDCl 3 ,300MHz):δ8.61(d,J=9.0Hz,1H,Ar-H),8.23-8.20(m,3H,Ar-H),8.16(s,1H,Ar-H),7.72(t, J=6.6Hz, 1H, Ar-H)...
Embodiment 2
[0049] Synthesis of 2-phenyl-4-ferrocenylquinoline
[0050] Ferroceneacetylene (0.231g, 1.1mmol), benzaldehyde (0.106g, 1.0mmol), aniline (0.093g, 1.1mmol), trifluoromethanesulfonic acid (0.015g, 0.10mmol) and ethanol (1mL) The mixture was stirred and reacted at 80°C for 2 hours. The reaction mixture was cooled to room temperature, ethyl acetate was added, washed with saturated sodium bicarbonate solution and brine respectively, the organic phase was dried over anhydrous magnesium sulfate, and the solvent was distilled off from the filtrate under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography (silica gel: 200-300 mesh, eluent: ethyl acetate / petroleum ether=1:20, v / v, R f =0.3) to obtain a red solid with a yield of 95%. The structural characterization data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com