Pathogenic detection kit for lentivirus of small ruminant
A technology for ruminants and kits, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., which can solve the problems of quarantine failure and timely diagnosis of virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, primer sequence: two primers were designed according to the DNA sequence of the disclosed small ruminant lentivirus in the GeneBanK of NCBI, P1 is the upstream primer; P2 is the downstream primer, and carried out relevant parameter analysis, see Table 1, Entrust a biotechnology company to carry out the synthesis.
[0020] Table 1 Sequence and related parameters of the two sequences
[0021]
[0022] 2. Detection Kit
[0023] 2.1 Standards
[0024] Negative control standard: sterile pure water without nucleotide contamination, pH6.8-7.2.
[0025] Positive control standard: Use artificially synthesized DNA fragments at a concentration of 0.5 μg / mL.
[0026] Table 2 Positive Control Standard Nucleotide Sequence
[0027]
[0028] 2.2 Reagents
[0029] 1mL 2x PCR Reagent (KT207-01) and 0.3mL D2000DNA Marker (MD114), produced by Tiangen Biochemical Technology (Beijing) Co., Ltd.; 5mL sterile pure water without nucleotide contamination, pH6.8-7.2; Prim...
Embodiment 2
[0049] Embodiment 2, use PCR method
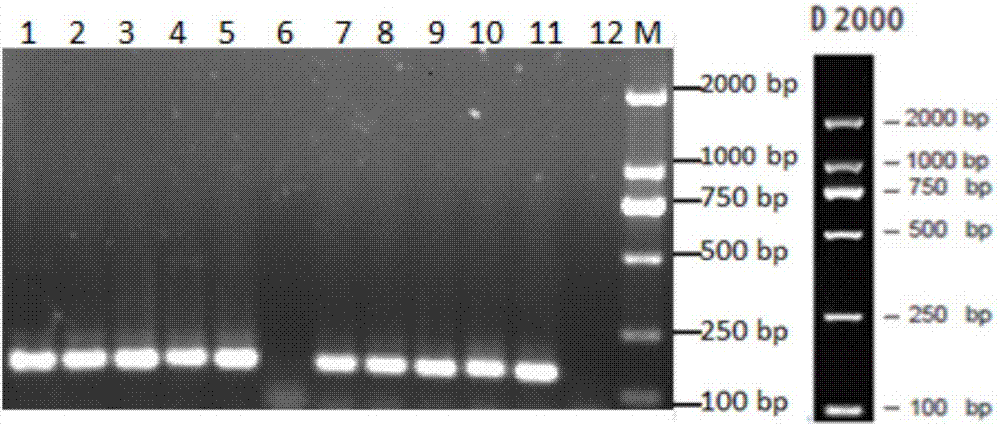
[0050] After caprine arthritis-encephalitis virus (CAEV) infects goats, the viral genetic material RNA is integrated into the host cell genome. By extracting the total DNA of white blood cells in goat blood, the established PCR method is used to detect the infection of goats with CAEV.
[0051] 1.1 Materials
[0052] Four copies of goat anticoagulant blood were screened by IDEXX CAEV / MVV Total Antibody Screening Kit (product number: CVT1135T), and the antibody was positive.
[0053] 1.2 Test method
[0054] The detection steps are as follows:
[0055] (1) Extraction of blood genome
[0056] Use the "Blood Genomic DNA Extraction Kit (0.1-1ml) (DP348)" produced by Tiangen Biochemical Technology (Beijing) Co., Ltd. for whole blood genome extraction;
[0057] (2) Carry out according to the PCR method in the above-mentioned technical scheme.
[0058] 2. Results
[0059] Screened by IDEXX CAEV / MVV Total Antibody Screening Kit, the serum anti...
Embodiment 3
[0061] Embodiment 3, use PCR method to detect sheep to be infected with MVV
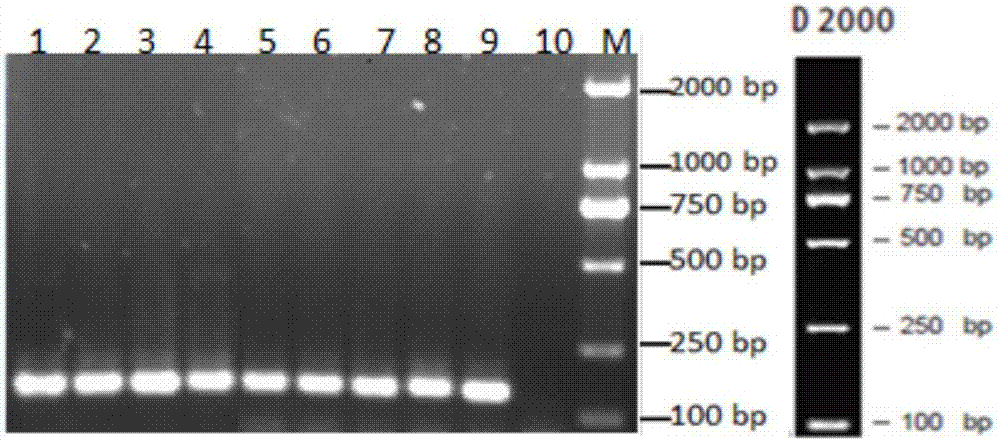
[0062] After sheep are infected by Medy-Visna virus (MVV), the viral genetic material RNA is integrated into the host cell genome. By extracting the total DNA of white blood cells in sheep blood, the established PCR method is used to detect the infection of sheep with MVV.
[0063] 1.1 Materials
[0064] Sheep anticoagulant was screened by IDEXX CAEV / MVV total antibody screening kit (product number: CVT1135T), and the antibody was positive.
[0065] 1.2 Test method
[0066] The detection steps are as follows:
[0067] (1) Extraction of blood genome
[0068] Use the "Blood Genomic DNA Extraction Kit (0.1-1ml) (DP348)" produced by Tiangen Biochemical Technology (Beijing) Co., Ltd. for whole blood genome extraction;
[0069] (2) Carry out according to the PCR method in the above-mentioned technical scheme.
[0070] 2. Results
[0071] Screened by IDEXX CAEV / MVV Total Antibody Screening Kit, 9 samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com