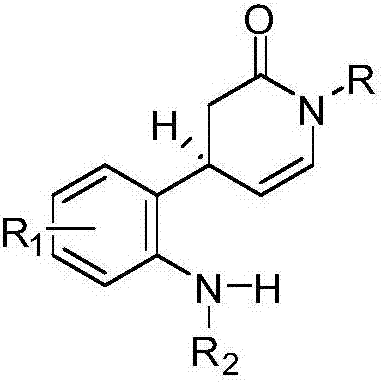
Synthetic methods for series of monoterpenoid indole alkaloid skeletons based on free radical tandem reactions and natural products
A nitrobenzenesulfonyl, selected technology, applied in the field of photocatalytic free radical tandem reaction technology to synthesize a series of monoterpene indole alkaloid skeletons, can solve the problems of high cost, cumbersome routes, and poor versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
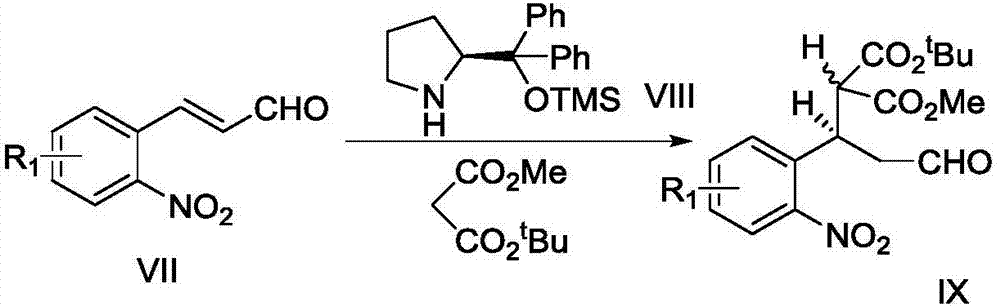
[0065] Embodiment 1: the preparation of compound IX
[0066]
[0067] Compound VII (10.0g, 0.056mol, 1.0equiv.), tert-butylmethyl maleate (12.3ml, 0.074mol, 1.3equiv.) and (2S)-2-[diphenyl[(trimethylsilyl Ester)oxy]methyl]-pyrrolidine VIII (3.7g, 0.011mol, 0.2equiv.) was placed in a 250mL round bottom flask, and 100mL of ethanol was added and stirred. After reacting at room temperature for 16 h, the solvent was drained. Column chromatography (petroleum ether: ethyl acetate, 5:1, v / v) gave two diastereoisomers IX-a and IX-b (total 15.1g), yield 76%.
[0068] The detection data of compound IX-a and IX-b are as follows: IX-a: TLC (petroleum ether: ethyl acetate, 5:1v / v): R f =0.30; 1 H NMR (400MHz, CDCl 3 ):δ9.68(s,1H),7.81(d,J=8.8Hz,1H),7.55(t,J=7.2Hz,1H),7.46(d,J=7.6Hz,1H),7.39(t ,J=8.4Hz,1H),4.56–4.50(m,1H), 3.83(d,J=9.2Hz,1H),3.57(s,3H),3.12–2.97(m,2H),1.43(s, 9H); 13 C NMR (100MHz, CDCl 3 ): δ199.6, 167.9, 166.5, 150.3, 134.8, 132.8, 129.3, 128.2, 124.7, 83.2, 57....
Embodiment 2
[0068] The detection data of compound IX-a and IX-b are as follows: IX-a: TLC (petroleum ether: ethyl acetate, 5:1v / v): R f =0.30; 1 H NMR (400MHz, CDCl 3 ):δ9.68(s,1H),7.81(d,J=8.8Hz,1H),7.55(t,J=7.2Hz,1H),7.46(d,J=7.6Hz,1H),7.39(t ,J=8.4Hz,1H),4.56–4.50(m,1H), 3.83(d,J=9.2Hz,1H),3.57(s,3H),3.12–2.97(m,2H),1.43(s, 9H); 13 C NMR (100MHz, CDCl 3 ): δ199.6, 167.9, 166.5, 150.3, 134.8, 132.8, 129.3, 128.2, 124.7, 83.2, 57.2, 52.6, 46.6, 33.7, 27.8, 27.8, 27.8; IR (neat): v max =1727,1355,845,749 cm -1 ;HRMS(m / z):[M+Na] + calcd.forC 17 h 21 NNaO 7 ,374.1210; found 374.1210; [α] D 25 = +63.4 (c 0.20, MeOR). IX-b: TLC (petroleum ether: ethyl acetate, 5:1v / v): R f =0.26; 1 H NMR (400 MHz, CDCl 3 ):δ9.68(s,1H),7.82(d,J=8.4Hz,1H),7.55(t,J=8Hz,1H),7.46 (d,J=7.6Hz,1H),7.38(t, J=7.6Hz, 1H), 4.53–4.47(m, 1H), 3.87(d, J= 9.6Hz, 1H), 3.73(s, 3H), 3.08–2.94(m, 2H), 1.20(s, 9H ); 13 C NMR (100MHz, CDCl 3 ): δ199.6, 168.4, 165.9, 150.2, 135.0, 132.9, 129.5, 128.2, 124.7, 82.8...
Embodiment 3
[0072] Embodiment 3: the preparation of compound I-a~I-j
[0073]
[0074] Take the preparation of I-a as an example to illustrate:
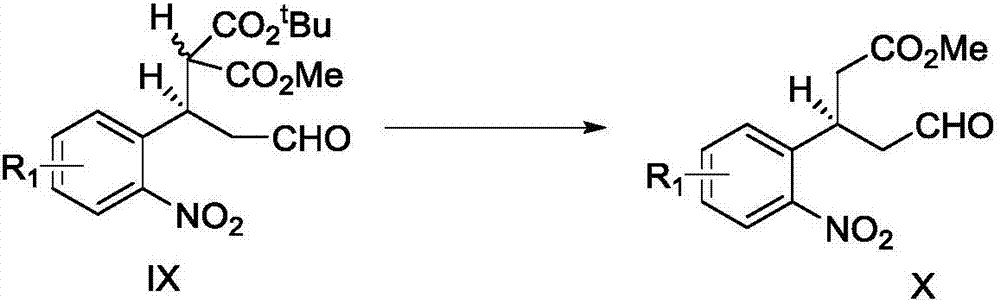
[0075] Compound X (890mg, 3.55mmol, 1.0equiv.), 3-buten-1-amine (324μL, 3.91mmol, 1.1equiv.), glacial acetic acid (406μL, 7.10mmol, 2.0equiv.) and The mixture of molecular sieves (powder, 890mg) was dissolved in toluene (178mL) and heated to 80°C. After maintaining the temperature and stirring for 8h, the molecular sieves were filtered off, washed with dichloromethane, and the solvent was drained. The crude product was purified by column chromatography (petroleum ether:ethyl acetate, 5:1, v / v) to obtain XI-a (704mg), with a yield of 73%. Compound XI-a (700mg, 2.57mmol, 1.0equiv.) was dissolved in methanol (35mL), and activated zinc powder (1.8g, 0.028mol, 11.0equiv.) and ammonium formate (1.6g, 0.026mol, 10.0equiv.) were added .). The solid was filtered off and the filtrate was sucked dry. The residue was dissolved with dichloromethane,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com