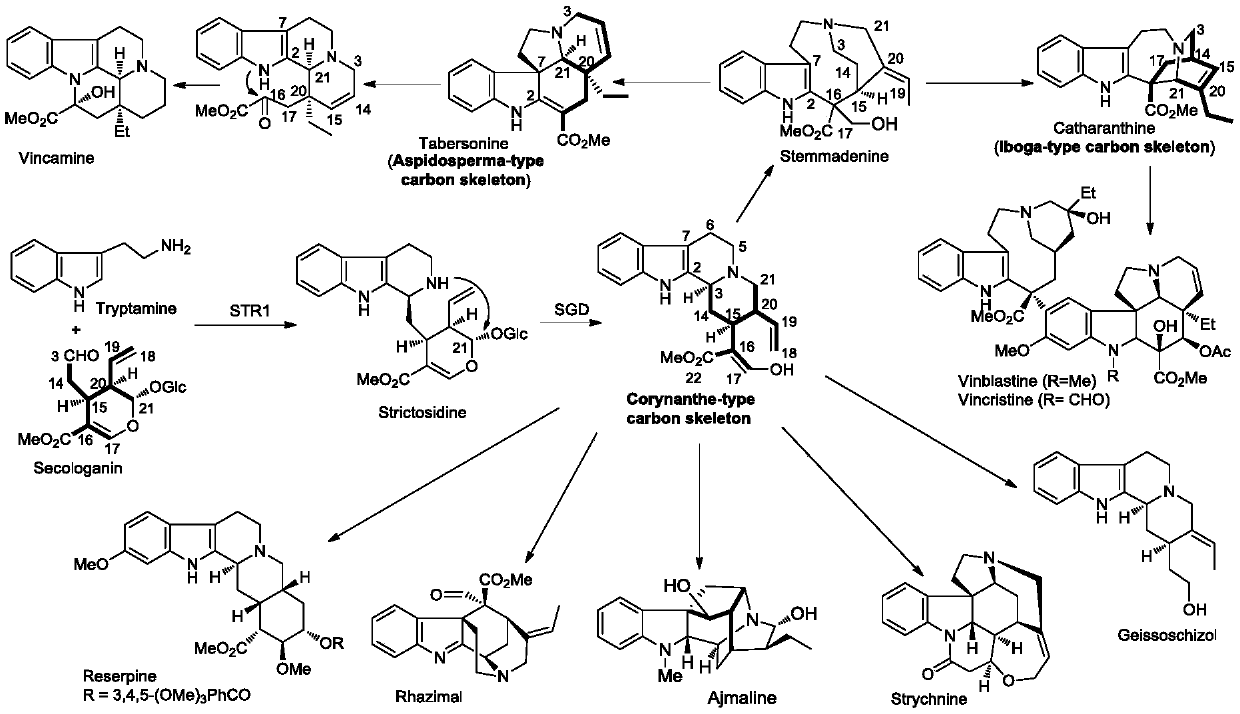
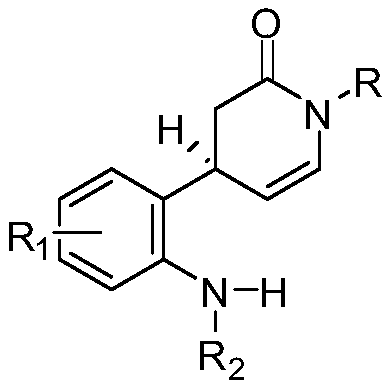
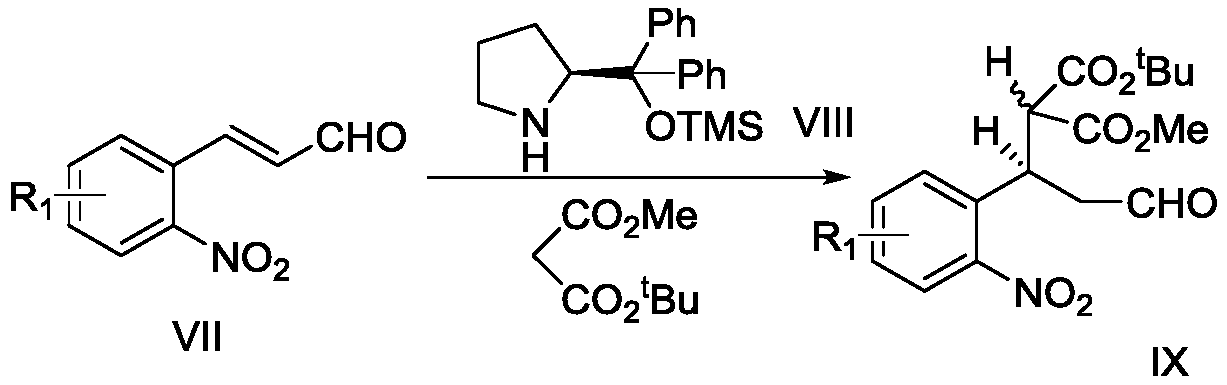
A method for the synthesis of a series of monoterpene indole alkaloid skeletons and natural products based on free radical tandem reactions
A nitrobenzenesulfonyl, selected technology, applied in the field of photocatalytic free radical tandem reaction technology to synthesize a series of monoterpene indole alkaloid skeletons, can solve the problems of poor versatility, high cost, cumbersome routes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of compound IX
[0066]
[0067] Compound VII (10.0g, 0.056mol, 1.0equiv.), tert-butyl methyl maleate (12.3ml, 0.074mol, 1.3equiv.) and (2S)-2-(diphenyl[(trimethylsilyl) Ester)oxy]methyl]-pyrrolidine VIII (3.7g, 0.011mol, 0.2equiv.) was placed in a 250mL round bottom flask, and 100mL ethanol was added and stirred. After 16h reaction at room temperature, the solvent was drained. Column chromatography (petroleum ether: ethyl acetate, 5:1, v / v) obtained two diastereomers IX-a and IX-b (total 15.1g), the yield was 76%.
[0068] The detection data of compounds IX-a and IX-b are as follows: IX-a: TLC (petroleum ether: ethyl acetate, 5:1 v / v): R f = 0.30; 1 H NMR(400MHz, CDCl 3 ): δ9.68(s,1H), 7.81(d,J=8.8Hz,1H), 7.55(t,J=7.2Hz,1H), 7.46(d,J=7.6Hz,1H), 7.39(t ,J=8.4Hz,1H),4.56–4.50(m,1H),3.83(d,J=9.2Hz,1H),3.57(s,3H),3.12–2.97(m,2H),1.43(s, 9H); 13 C NMR(100MHz, CDCl 3 ):δ199.6,167.9,166.5,150.3,134.8,132.8,129.3,128.2,124.7,83.2,57.2,52.6,46.6,33.7,27.8,2...
Embodiment 2
[0069] Example 2: Preparation of Compound X
[0070]
[0071] After the mixtures IX-a and IX-b (40.2 g, 0.114 mol, 1.0 equiv.) were dissolved in dichloromethane (333 mL), trifluoroacetic acid (267 mL) was slowly added. After 3h reaction at room temperature, the solvent was drained. After the residue was dissolved in dichloromethane (200 mL), the solvent was drained. This operation was repeated three times to remove residual trifluoroacetic acid. After the crude product was dissolved in toluene (333 mL), triethylamine (23.7 mL, 0.170 mol, 1.5 equiv.) was added. The reaction was refluxed for 2h. After the solvent was drained, water (100mL) and saturated brine (200mL) were added. It was extracted three times with ethyl acetate (3×500mL), the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was drained, and purified by column chromatography (petroleum ether: ethyl acetate, 3:1, v / v) to obtain compound X ( 17.1g), the yield is 60%. HPLC (AD-H, isopro...
Embodiment 3
[0073] Example 3: Preparation of Compound I-a~I-j
[0074]
[0075] Take the preparation of I-a as an example:
[0076] Compound X (890mg, 3.55mmol, 1.0equiv.), 3-butene-1-amine (324μL, 3.91mmol, 1.1equiv.), glacial acetic acid (406μL, 7.10mmol, 2.0equiv.) and After the mixture of molecular sieves (powder, 890 mg) was dissolved in toluene (178 mL), it was heated to 80°C. After stirring for 8h at this temperature, the molecular sieve was filtered off, washed with dichloromethane, and the solvent was drained. The crude product was purified by column chromatography (petroleum ether: ethyl acetate, 5:1, v / v) to obtain XI-a (704 mg) with a yield of 73%. After compound XI-a (700mg, 2.57mmol, 1.0equiv.) was dissolved in methanol (35mL), activated zinc powder (1.8g, 0.028mol, 11.0equiv.) and ammonium formate (1.6g, 0.026mol, 10.0equiv.) .). The solid was filtered off, and the filtrate was drained. The residue was dissolved with dichloromethane and washed with saturated aqueous ammoniu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com