Therapeutic agent for dry eye syndrome accompanied by Sjogren's syndrome
A technology for Sjögren's syndrome and dry eye syndrome, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as non-recognition, susceptibility to infection, and increased intraocular pressure, and achieve the goal of improving epithelial damage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Improvement effect on MRL / Lpr mice (Sjogren's syndrome model mice)
[0046] 1. Test method
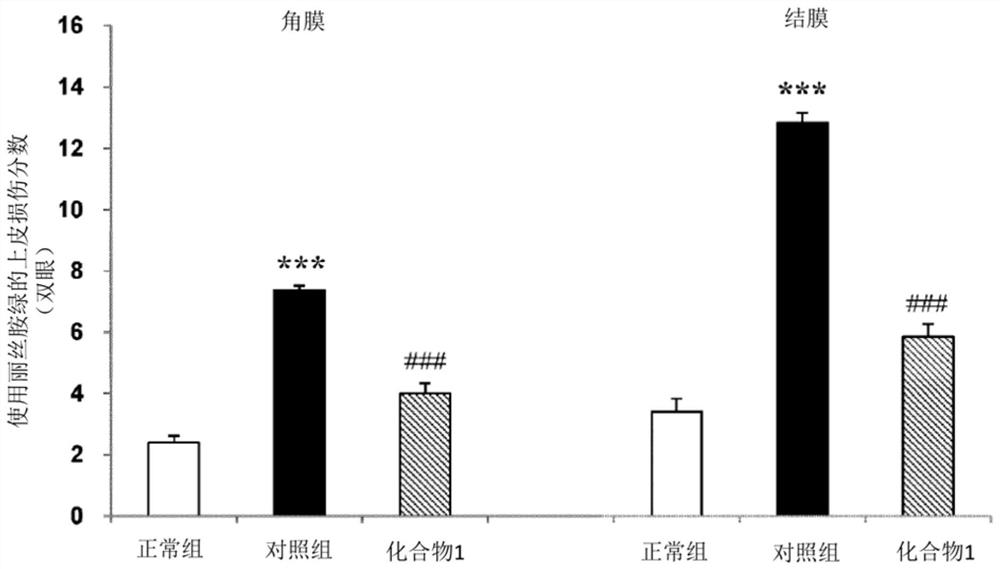
[0047] 18-week-old MRL / Lpr mice (Jabs DAPrendergast RA. Murine models of Sjogren's syndrome. Adv Exp Med Biol. 1994, 350, p. 623-30) known as a model animal of Sjogren's syndrome, Both eyes were administered 1% (w / v) sodium ozagrel eye drops or citric acid buffer solution as a control, 4 times a day (3 hours interval) with 5 μL per eye, for 14 days (13-14 days in each group). example). In addition, mice from the Institute of Cancer Research (ICR, Institute of Cancer Research) were used as a normal group (Normal) (15 cases), and citrate buffer was similarly administered to the normal group by eye drops. On the day after the last eyedrop administration, 1 μL of 1% Lissamine Green solution was instilled in both eyes under pentobarbital anesthesia, and the degree of damage to the cornea and conjunctiva was scored. Regarding the corneal damage score, the case where punctate staini...
Embodiment 2
[0051] Improvement effect on dry eye patients with Sjogren's syndrome
[0052] 1. Test method
[0053] For 65 patients with dry eye syndrome accompanied by Sjogren's syndrome, placebo (21 cases), or 1% (w / v) (22 cases) or 0.5% was repeatedly administered to both eyes with 1 drop once, 4 times a day (w / v) (22 cases) of sodium ozagrel eye drops for 8 weeks.
[0054] It should be noted that the results of this test were carried out on patients with dry eye syndrome, referring to the conditions of the revised diagnostic criteria for Sjogren's syndrome (1999) of the Research Group of the Ministry of Health, Labor and Welfare of Japan, and extracting dry eye with Sjogren's syndrome obtained from the analysis of patients with the disease.
[0055] 2. Evaluation items
[0056] The total score of lissamine green conjunctival staining (the damage degree of the 6 regions is scored on a scale of 0 to 3 points), the total score of the fluorescein corneal staining degree (the damage degr...
Embodiment 3
[0061] Study on usage and dosage of dry eye syndrome accompanied by Sjogren's syndrome
[0062] 1. Analysis method
[0063] In the results of the following clinical trials A and B for dry eye patients, dry eye patients with Sjögren's syndrome were extracted on the same basis as in Example 2. The effects of placebo (10 cases), or 1% (w / v) (7 cases) or 2% (w / v) (13 cases) of Ozagrel sodium eye drops on those associated with Sjogren's syndrome The effect of dry eye patients was analyzed.
[0064] 2. Test content
[0065] Clinical Trial A:
[0066] For patients with dry eye syndrome, placebo, or 0.5% (w / v), 1% (w / v) or 2% (w / v) is repeatedly administered to both eyes with 1 drop once, 4 times a day The concentration of ozagrel sodium eye drops for 4 weeks.
[0067] Clinical Trial B:
[0068] For patients with dry eye syndrome, placebo or ozagrel sodium drops at a concentration of 1% (w / v) or 2% (w / v) were repeatedly administered to both eyes 1 drop at a time, 4 times a day ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com