Supermolecule cage complex as well as preparation method and application thereof
A technology of complexes and supramolecules, applied in organic chemistry methods, zinc organic compounds, cobalt organic compounds, etc., can solve problems such as residence and inclusion research, and achieve the effects of wide sources of raw materials and reagents, low price, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
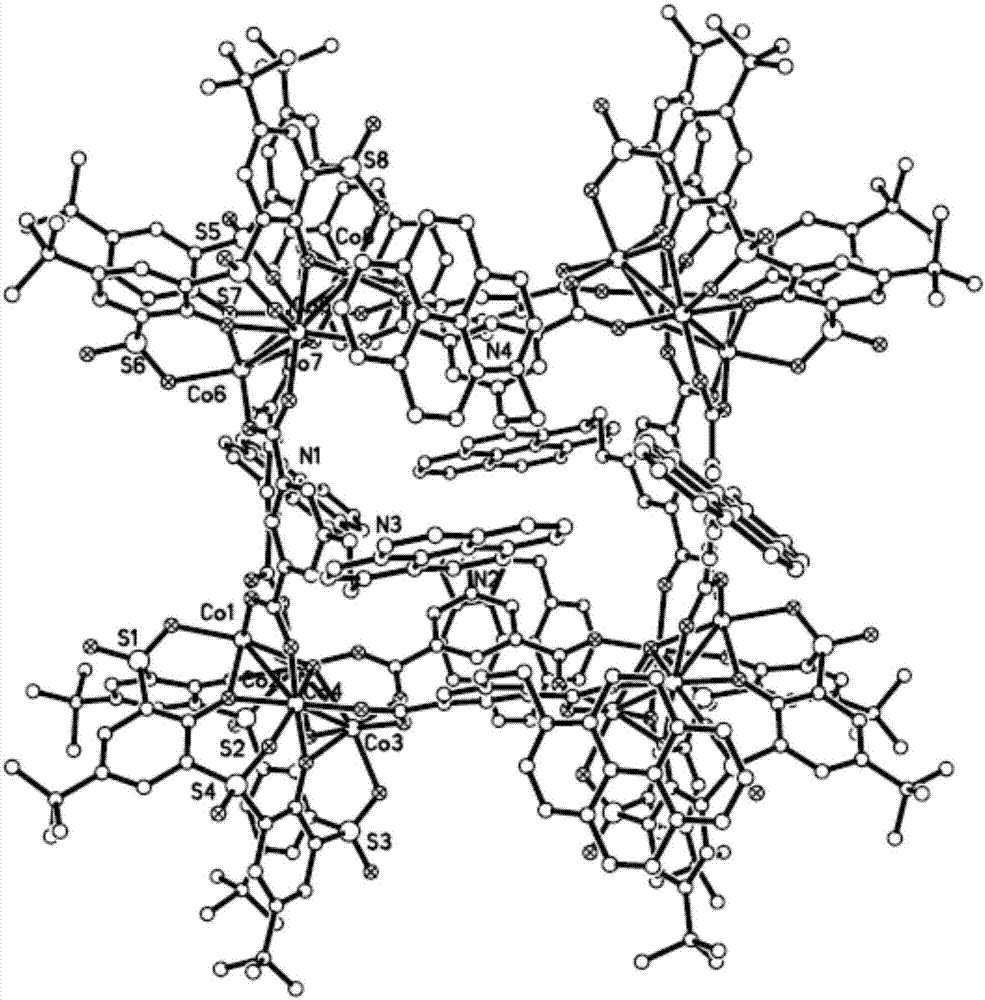
[0065] Preparation of supramolecular cage complex {[Co 4 (μ 4 -H 2 O)(TBSC)] 4 [DC] 8}
[0066] (where TBSC stands for 5,11,17,23-tetra-tert-butyl-sulfonyl bridged calix[4]arene ligand, DC stands for 5-(pyrenyl-1-methyl)aminoisophthalic acid ligand)
[0067] Take Co(NO 3 ) 2 ·6H 2 O (72.80mg, 0.25mmol), 5-(pyrenyl-1-methyl)aminoisophthalic acid (67.50mg, 0.17mmol) and H 4 TBSC (42.50 mg, 0.05 mmol) was dissolved in 5 mL of N,N'-dimethylformamide (DMF) and 4 mL of methanol mixed solvent, heated to 100 °C at a heating rate of 0.5 °C / min, and the temperature was maintained at 100 After reacting at ℃ for 24 hours, the temperature was slowly lowered to obtain red crystals. Yield: 85%.
[0068] The product was characterized by X-ray single crystal diffraction, and the specific results are as follows:
[0069] Table 1. {[Co 4 (μ 4 -H 2 O)(TBSC)] 4 [DC] 8} crystallographic parameters
[0070]
[0071] Above-mentioned data shows that present embodiment has obtained ...
preparation example 2
[0074] Preparation of supramolecular cage complex {[Zn 4 (μ 4 -H 2 O)(TBSC)] 4 [DC] 8}
[0075] (where TBSC stands for 5,11,17,23-tetra-tert-butyl-sulfonyl bridged calix[4]arene ligand, DC stands for 5-(pyrenyl-1-methyl)aminoisophthalic acid ligand)
[0076] Take Zn(NO 3 ) 2 ·6H 2 O (74.50mg, 0.25mmol), 5-(pyrenyl-1-methyl)aminoisophthalic acid (67.50mg, 0.17mmol) and H 4 TBSC (42.50 mg, 0.05 mmol) was dissolved in 5 mL of N,N'-dimethylformamide (DMF) and 4 mL of methanol mixed solvent, heated to 100 °C at a heating rate of 0.5 °C / min, and the temperature was maintained at 100 After reacting at ℃ for 24 hours, the temperature was lowered slowly to obtain yellow crystals. Yield: 75%.
[0077] The product is characterized by X-ray single crystal diffraction:
[0078] Table 2. {[Zn 4 (μ 4 -H 2 O)(TBSC)] 4 [DC] 8} crystallographic parameters
[0079]
[0080] Above-mentioned data shows that present embodiment has obtained target product {[Zn 4 (μ 4 -H 2 O)(T...
Embodiment 1
[0082] The interaction of the supramolecular cage complex prepared in Preparation Example 1 with acid and base in chloroform solution was investigated. The concentration of the supramolecular cage complex is 6×10 -6 M, the concentrations of trifluoroacetic acid and triethylamine were 6×10 -3 M. Detect its fluorescence emission intensity, the fluorescence emission excitation wavelength is 366nm, the result is as follows figure 2 shown.
[0083] figure 2 It is the fluorescence emission spectrum of the chloroform solution of the supramolecular cage complex of Example 1 of the present invention under acid and alkali conditions. Depend on figure 2 It can be seen that the maximum absorption peak intensity of the supramolecular cage complex in Example 1 will be greatly enhanced under acidic conditions, while the fluorescence will be quenched under alkaline conditions. Moreover, the acid-base regulated fluorescent switching process is completely reversible and can be cycled m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com