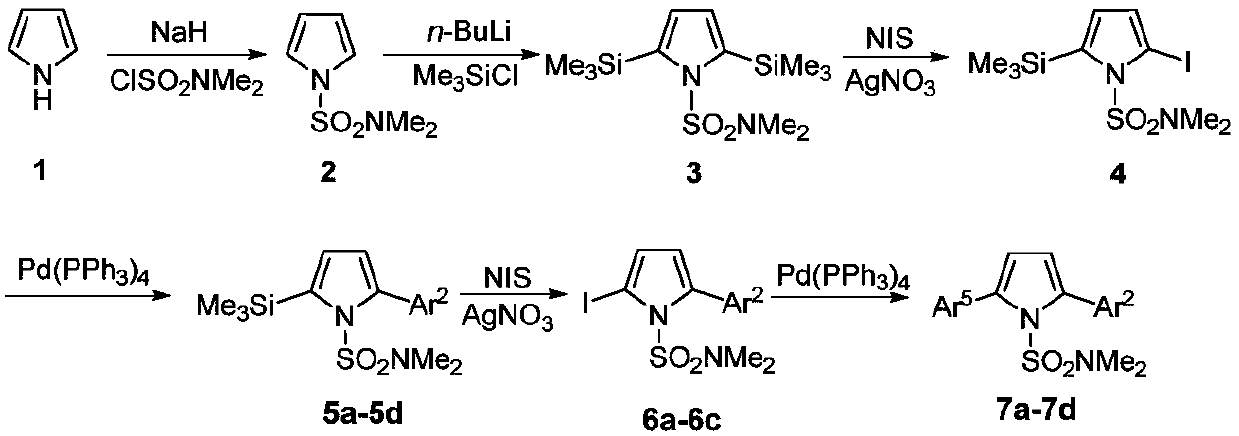
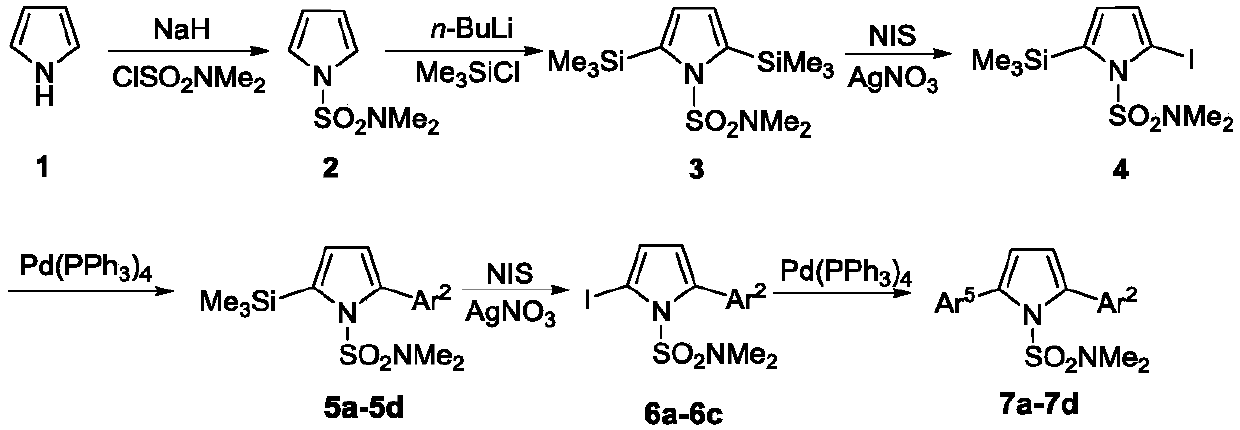
A kind of regioselective synthesis method of 2,5-disubstituted pyrrole
A regioselective, disubstituted pyrrole technology, applied in organic chemistry and other directions, can solve the problems of limited substituents, complex product types, and high toxicity, and achieves the effect of good universality and simplified synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The first step, the synthesis of compound 2
[0036]Under nitrogen protection, add NaH (1.73g, 43.2mmol), DMF (50 mL) in 250mL two-necked flask; Add pyrrole (2.42g, 36mmol) and DMF (20mL) in 100mL two-necked flask, stir in ice bath Add it dropwise to the DMF solution of NaH washed with n-hexane, stir for 1h; add ClSO dropwise 2 NMe 2 (3.9mL, 36mmol), react at 0°C for 2h. Add an appropriate amount of ice water to the two-neck flask, extract with ether, collect the organic phase, wash with water and saturated brine, and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure and then separated by column. The developer was n-hexane:ether (10:1) to obtain 4.39 g of white solid with a yield of 70.0%. mp:61-62℃; 1 H NMR (500MHz, CDCl 3 )δ2.79(s,6H),6.31(t,1H,J=2Hz),7.09(t,1H,J=2Hz); 13 C NMR (125MHz, CDCl 3 )δ38.3, 111.7, 120.9. HRMS (ESI-TOF) for C 6 h 10 N 2 o 2 S[M+H] + :calcd,175.0536.found 175.0536.
[0037] The second step, the...
Embodiment 2
[0078] The first step, the synthesis of compound 2
[0079] Under nitrogen protection, NaH (1.44g, 36mmol) and DMF (50 mL) were added to a 250mL two-necked flask; pyrrole (2.42g, 36mmol) and DMF (20mL) were added to a 100mL two-necked flask, and the Add dropwise to the DMF solution of NaH washed with n-hexane, stir for 1h; add ClSO dropwise 2 NMe 2 (3.9mL, 36mmol), react at 0°C for 2h. Add an appropriate amount of ice water to the two-neck flask, extract with ether, collect the organic phase, wash with water and saturated brine, and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure and then separated by column. The developer was n-hexane:ether (10:1) to obtain 4.39 g of white solid with a yield of 80%.
[0080] The second step, the synthesis of compound 3
[0081] Under nitrogen protection, THF (60mL), TMP (2,2,6,6-tetramethylpiperidine) (8.05mL, 47.4mmol) were added to a 250mL two-necked flask, and n- BuLi (14.2mL / 2.5M, 36mmol), reacte...
Embodiment 3
[0110] The first step, the synthesis of compound 2
[0111] Under nitrogen protection, NaH (4.32g, 108mmol) and DMF (50 mL) were added to a 250mL two-necked flask; pyrrole (2.42g, 36mmol) and DMF (20mL) were added to a 100mL two-necked flask, and the Add dropwise to the DMF solution of NaH washed with n-hexane, stir for 3h; add ClSO dropwise 2 NMe 2 (7.8mL, 72mmol), react at room temperature for 5h. Add an appropriate amount of ice water to the two-neck flask, extract with ether, collect the organic phase, wash with water and saturated brine, and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure and then separated by column. The developer was n-hexane:ether (10:1) to obtain 4.39 g of white solid with a yield of 70%.
[0112] The second step, the synthesis of compound 3
[0113] Under nitrogen protection, THF (60mL), TMP (2,2,6,6-tetramethylpiperidine) (8.05mL, 47.4mmol) were added to a 250mL two-necked flask, and n- BuLi (28.5mL / 2.5M, 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com