2-aminoimidazopyridine derivatives and their preparation and application
A technology of azopyridines and imidazopyridines, applied in the field of 2-aminoimidazopyridine derivatives and their preparation, can solve the problems such as limited clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
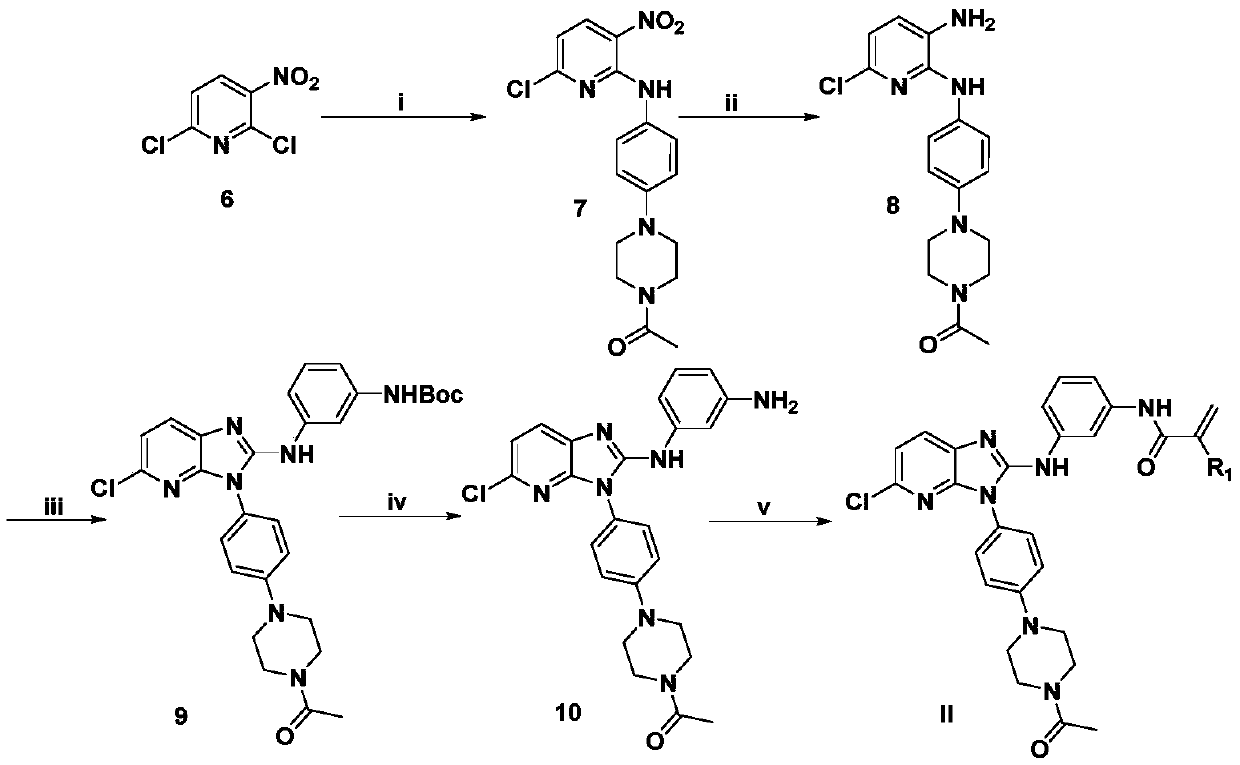
[0056] Example 1: N-(3-(5-chloro-2-((4-(4-methylpiperazine)phenyl)amino)-3H-[4,5-b]imidazopyridine)phenyl) Acrylamide;
[0057]
[0058] Step 1: Mix 2,6-dichloro-3-nitropyridine (1.92g, 10mmol) with tert-butyl (3-aminophenyl)carbamate (2.08g, 10mmol) and potassium carbonate (1.38g, 10mmol) Dissolve in ethanol (25ml) and react overnight. After the reaction was finished, the solid in the system was filtered with suction to obtain a red solid (3g), the product yield was 82%, m.p.: 157.7-158.6°C; 1 H NMR(500MHz,DMSO)δ10.06(s,1H),9.48(s,1H),8.53(d,J=8.5Hz,1H),7.70(s,1H),7.27-7.24(m,3H) ,7.00(d,J=8.5Hz,1H),1.48(s,9H).HRMS(ESI):m / z calcd for(C 16 h 17 ClN 4 o 4 +H) + :365.1011; found: 365.1015.
[0059] Step 2: Dissolve the product obtained in Step 2 (3g, 8.25mmol), sodium borohydride (1.32g, 33mmol) and nickel chloride (3.9g, 16.5mmol) in dichloromethane (20mL), and cool to 0°C React at -2°C for 1 hour. After the reaction was complete, water was added, extracted with di...
Embodiment 2
[0064] Example 2: N-(3-(5-chloro-2-((4-(4-methylpiperazine)phenyl)amino)-3H-[4,5-b]imidazopyridine)phenyl) -2-methacrylamide
[0065] Referring to the method of Example 1, except that the 2-chloroacryloyl chloride in step 5 was replaced by 2-fluoroacryloyl chloride, after the reaction was completed, the crude product was concentrated to dryness under reduced pressure to obtain a crude product, which was purified by column chromatography (DCM / MeOH=75:1 ) to obtain white solid 0.9g, yield 40%, m.p.:>200℃, 1 H NMR (500MHz, DMSO) δ7.95(d, J=8.2Hz, 1H), 7.92(s, 1H), 7.75(d, J=8.1Hz, 1H), 7.66(d, J=8.6Hz, 2H ),7.59(t,J=8.1Hz,1H),7.26(d,J=7.4Hz,1H),7.19(d,J=8.1Hz,1H),6.99(d,J=9.0Hz,2H), 5.85(s,1H),5.56(s,1H),3.47(m,4H),3.14(m,4H),2.80(s,3H),1.96(s,3H).HRMS(ESI):m / z calcd for (C 27 h 28 ClN 7 O+H) + :502.2117; found: 502.2115.
Embodiment 3
[0066] Example 3: N-(3-(5-chloro-2-((4-(4-methylpiperazine)phenyl)amino)-3H-[4,5-b]imidazopyridine)phenyl) -2-fluoroacrylamide
[0067] Referring to the method of Example 1, except that the 2-chloroacryloyl chloride in step 5 was replaced with 2-fluoroacryloyl chloride, after the reaction, concentrated to dryness under reduced pressure to obtain the crude product, which was purified by column chromatography (DCM / MeOH=75: 1) 1.0 g of white solid was obtained with a yield of 46%. m.p.:>200℃, 1 H NMR (500MHz, CDCl 3)δ8.57(s,1H),7.92(s,1H),7.71-7.67(m,2H),7.54(t,J=8.1Hz,1H),7.49(d,J=8.8Hz,2H), 7.29(d, J=7.8Hz, 1H), 7.12(d, J=8.2Hz, 1H), 6.92(d, J=8.8Hz, 2H), 6.47(s, 1H), 5.79(dd, J=47.2 ,3.3Hz,1H),5.24(dd,J=15.0,3.3Hz,1H),3.21-3.17(m,4H),2.61(m,4H),2.37(s,3H).HRMS(ESI):m / z calcd for (C 26 h 25 ClFN 7 O+H) + :506.1866; found: 506.1865.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com