5-aminopyrazole formamide derivative as BTK inhibitor, preparation method and pharmaceutical composition thereof
A technology of aminopyrazolecarboxamide and compound is applied in the fields of 5-aminopyrazolecarboxamide derivative derivatives, preparation methods and pharmaceutical compositions thereof, and can solve the problems of increased burden on patients, influence on drug efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
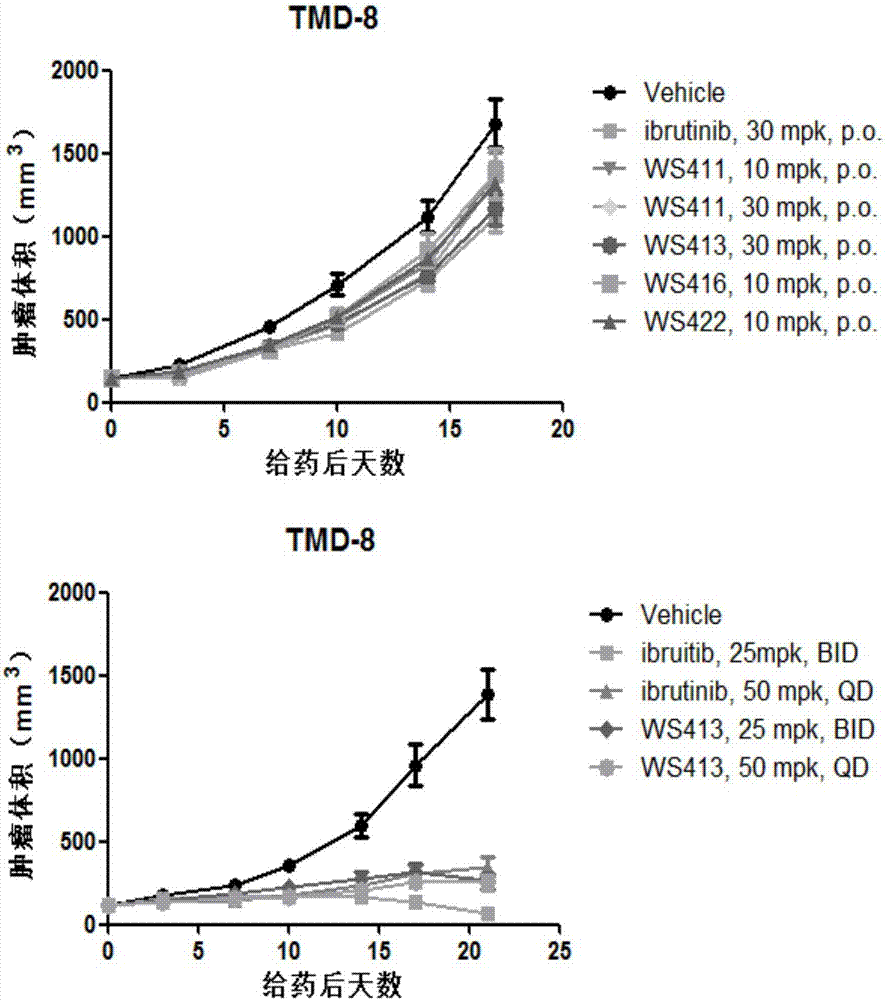
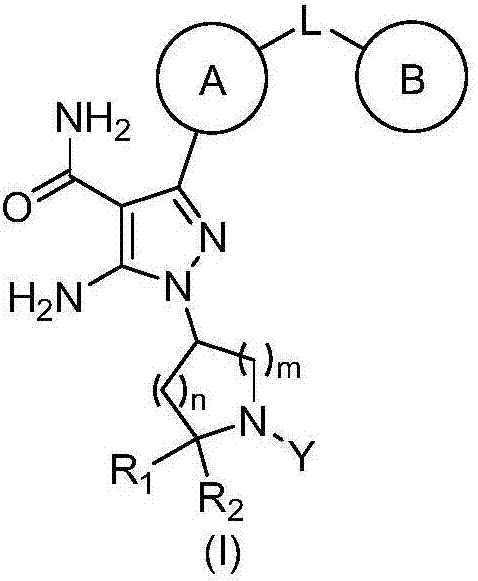
Image
Examples
Embodiment 1
[0114] 5-amino-3-(4-((5-chloropyridin-2-yl)oxy)phenyl)-1-(1-cyano-6-methylpiperidin-3-yl)-1H-pyridine Preparation of azole-4-carboxamide (WS-400)
[0115]
[0116] The preparation of the first step 6-methylpiperidin-3-ol
[0117] 5-Hydroxy-2-methylpyridine (1 g, 9 mmol) was dissolved in acetic acid (20 mL), then platinum dioxide (0.25 g) was added. The reaction mixture was shaken overnight under a hydrogen atmosphere at a pressure of 50 psi. The solution was filtered off and spun dry to give crude 6-methylpiperidin-3-ol.
[0118] The preparation of second step 5-hydroxyl-2-methylpiperidine-1-carboxylic acid benzyl ester
[0119] 6-Methylpiperidin-3-ol was dissolved in dichloromethane (100 mL), then triethylamine (7.5 eq) was added dropwise, followed by benzyloxycarbonyl chloride (2 g, 11.2 mmol). The reaction was complete after stirring for 16 hours. After the reaction solution was spin-dried, the residue was purified by silica gel column to obtain the desired product ...
Embodiment 2
[0152] 5-amino-3-(4-((5-chloropyridin-2-yl)oxy)phenyl)-1-((3R,6S)-1-cyano-6-methylpiperidine-3- base)-1H-pyrazole-4-carboxamide (WS-401) preparation
[0153]
[0154] The product of the reaction in the fourteenth step of Example 1 can be obtained by chiral resolution to obtain the compound of Example 2. The resolution conditions are: Supercritical fluid chromatography (ChiralPak AD 5μ, 21x 250 mm col, 27% methanol, 70mL / min). MS: m / z 452.2[M+1]
Embodiment 3
[0156]5-amino-3-(4-((5-chloropyridin-2-yl)oxy)phenyl)-1-((3S,6R)-1-cyano-6-methylpiperidine-3- base)-1H-pyrazole-4-carboxamide (WS-402) preparation
[0157]
[0158] The product of the fourteenth step reaction in Example 1 can obtain the compound of Example 3 after chiral resolution. The resolution conditions are: Supercritical fluid chromatography (ChiralPak AD 5μ, 21x 250 mm col, 27% methanol, 70mL / min). MS: m / z 452.2[M+1]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com