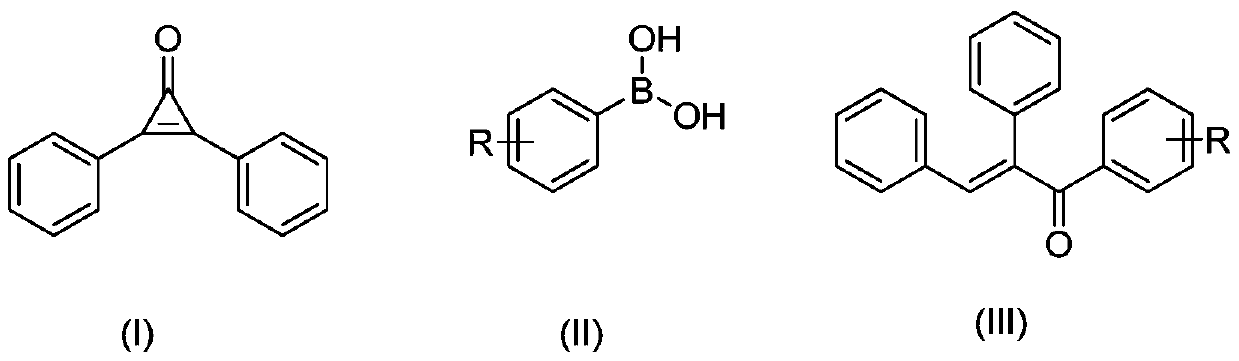
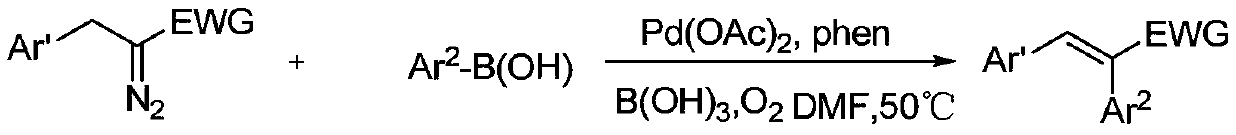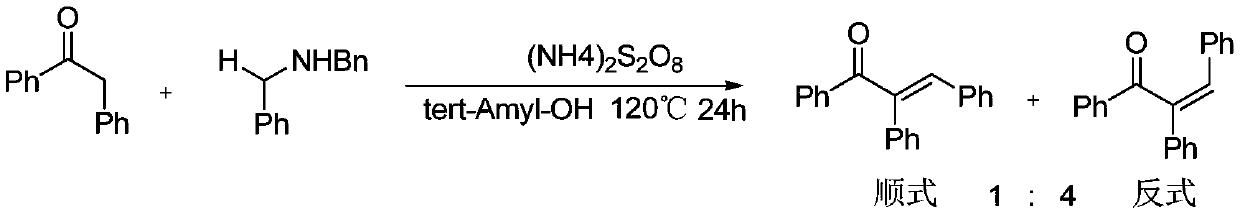Preparation method of α,β-diphenyl-1-arylpropenone compound
A technology for diphenylcyclopropenone and arylpropenone, which is applied in the field of organic compound synthesis, can solve the problems of insufficient mild reaction conditions, complicated operation and the like, and achieves the effects of simple operation, simple reaction operation and high tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] In the preparation method of the present invention, the reaction temperature is 0-30°C, non-limitingly, for example, it can be 0°C, 10°C, 20°C or 30°C
[0050] (6) Response time
[0051] In the preparation method of the present invention, the reaction time is not particularly limited. For example, the appropriate reaction time can be determined by detecting the residual percentage of the target product or raw material through liquid chromatography, which is usually 1-3 hours, non-limitingly for example 1 hour, 2 hours and 3 hours.
[0052] (7) Separation and purification
[0053] The mixture obtained after the reaction can be further separated and purified to obtain a purer final product. Those of ordinary skill in the art are well-known methods for separation and purification, for example, methods such as extraction, column chromatography, distillation, decantation, filtration, centrifugation, washing, evaporation, stripping and adsorption or a combination of at leas...
Embodiment 1
[0057]
[0058] At 30°C, 0.1mmol of diphenylcyclopropenone, 0.01mmol of Pd(PPh 3 ) 4 , 0.15mmol of phenylboronic acid was added to the reaction tube, and then pumped and filled with nitrogen three times. Under the nitrogen environment, 1ml of 1.4-dioxane was added, and the reaction was stirred at 30°C for 1 hour to obtain α,β-diphenyl- 1-Arylpropenone compounds.
[0059] Then add 10ml of ethyl acetate, then add silica gel powder, and obtain the product after separation by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1), the product is a yellow solid, and the yield is 92%;
[0060] The proton nuclear magnetic resonance spectrum data of gained product are as follows:
[0061] 1 H NMR (500MHz, CDCl 3 )δ7.87-7.86(d,J=5.0Hz 2H),7.56-7.53(m,1H),7.46-7.43(m,2H),7.36-7.33(m,2H),7.29-7.28(m,2H ),7.24-7.17(m,4H),7.10-7.08(d,J=10.0Hz2H);
[0062] The carbon nuclear magnetic resonance spectrum data of gained product are as follows:
[0063] 13 C NMR (125MH...
Embodiment 2
[0065]
[0066] At 0°C, 0.1 mmol of diphenylcyclopropenone, 0.001 mmol of PdCl 2 , 0.5mmol of 2-naphthylphenylboronic acid was added to the reaction tube, and then pumped and filled with nitrogen three times. Under nitrogen environment, 1ml of ethyl acetate was added and stirred at 0°C for 3 hours to obtain α,β-diphenyl-1 - arylpropenone compounds;
[0067] Then add 10ml of ethyl acetate, then add silica gel powder, and obtain the product after separation by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1), the product is a white solid, and the yield is 89%;
[0068] The proton nuclear magnetic resonance spectrum data of gained product are as follows:
[0069] 1 H NMR (500MHz, CDCl 3 )δ8.38(s,1H),7.87-7.95(m,4H),7.51-7.60(m,2H),7.30-7.38(m,1H),7.15-7.24(m,3H),7.11(d, J=10.0Hz 2H);
[0070] The carbon nuclear magnetic resonance spectrum data of gained product are as follows:
[0071] 13 C NMR (125MHz, CDCl 3 )δ 197.5, 141.0, 139.9, 136.6, 135.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com