Diarylmethylene disulfide compound as well as preparation method and application thereof
A technology for compounds and preparations, applied in the field of diarylmethylene disulfide compounds and their preparation, can solve the problems of limited treatment means and great harm of cerebral apoplexy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
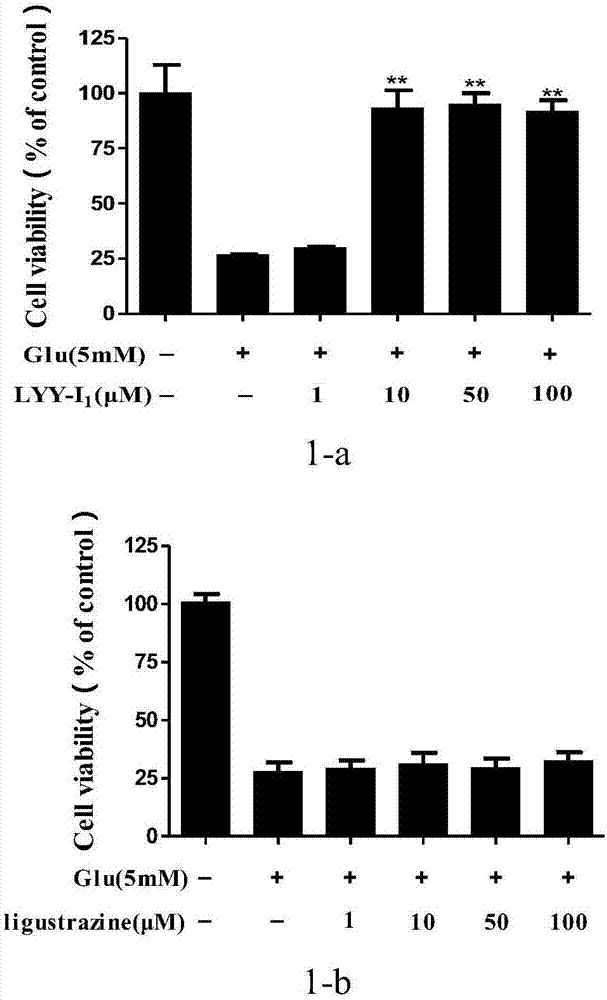
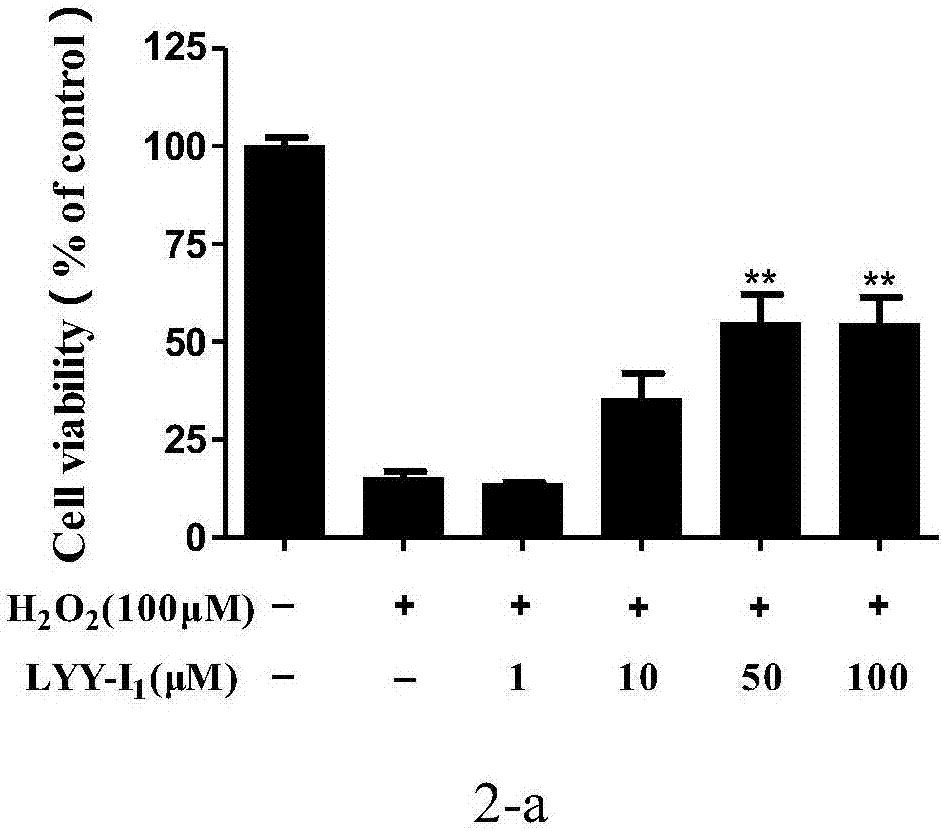
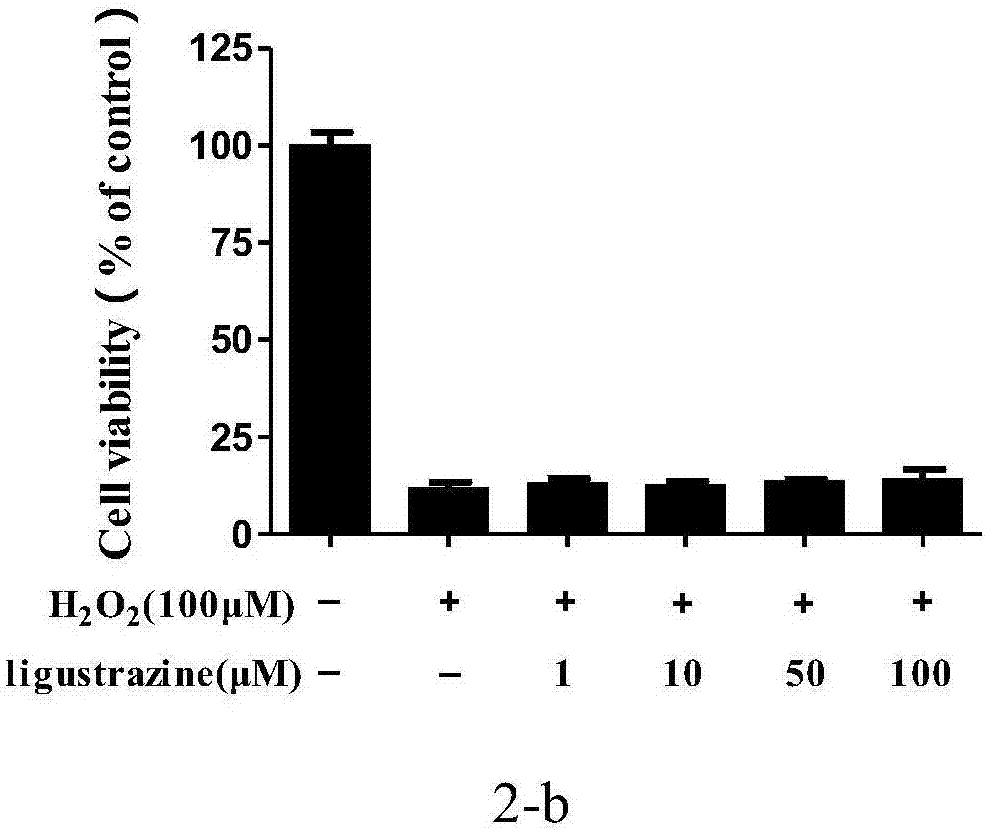
[0055] Example 1 1,2-bis[(3,5,6-trimethylpyrazin-2-yl)methyl]disulfide (LYY-Ι 1 )
[0056] 1-bromotetramethylpyrazine (0.210g, 0.97mmol), thiourea (0.111g, 1.45mmol), sodium carbonate (0.154g, 1.45mmol), manganese dioxide (0.084g, 0.97mmol) were added to a 100mL reaction flask , then add wet-PEG200 (20mL) (PEG200:H 2 O=10:1), heated to 40°C, and stirred for 3h. Cool to room temperature, add water (20mL), extract with ethyl acetate (20mL) three times, wash with water (50mL) three times, dry over anhydrous magnesium sulfate, filter with suction, column chromatography [eluent petroleum ether: ethyl acetate (v :v)=10:1], a yellow solid was obtained. Yield 45%. Mp: 73~75℃.
[0057] 1 H NMR (400MHz, CDCl 3 ), δ (ppm): 3.99 (s, 4H, CH 2 ),2.54(s,6H,CH 3 ),2.49(s,12H,CH 3 ).
[0058] 13 C-NMR (600MHz, CDCl 3 ), δ(ppm): 150.17, 148.94, 148.64, 146.61, 42.67, 21.62, 21.40, 21.09.
[0059] HR-MS:[M+H] + , Calcd: 335.1364, Found: 335.1360.
Embodiment 2
[0060] Example 2 1,2-dibenzyl disulfide (LYY-Ι 2 )
[0061] Reference compound LYY-Ι 1 The synthesis method is prepared from benzyl bromide and thiourea to obtain a white solid. Yield 57%. Mp: 64~68℃.
[0062] 1 H NMR (400MHz, CDCl 3 ,δ(ppm):7.35-7.32(m,2H,ArH),7.32-7.28(m,4H,ArH),7.24(dd,J=5.8,4.3Hz,4H,ArH),3.60(s,4H, CH 2 ).
[0063] 13 C-NMR (600MHz, CDCl 3 ), δ(ppm): 137.60, 129.65, 128.72, 127.66, 43.03.
[0064] HR-MS:[M+H] + , Calcd: 247.0615, Found: 247.0611.
Embodiment 3
[0065] Example 3 4,4'-Disulfanediyl bis(methylene)dibenzoate methyl ester (LYY-I 3 )
[0066] Reference compound LYY-Ι 1 The synthesis method is prepared from 4-(bromomethyl)methyl benzoate and thiourea to obtain a white solid. Yield 51%. Mp: 81~85℃.
[0067] 1H NMR (400MHz, CDCl 3 ), δ (ppm): 8.01–7.97 (m, 4H, ArH), 7.28 (d, J = 8.3Hz, 4H, ArH), 3.92 (s, 6H, OCH 3 ),3.62(s,4H,CH 2 ).
[0068] 13C-NMR (600MHz, CDCl 3 ), δ(ppm): 166.80, 142.61, 129.86, 129.41, 129.35, 52.21, 42.91.
[0069] HR-MS:[M+H] + , Calcd: 363.0725, Found: 363.0724.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com