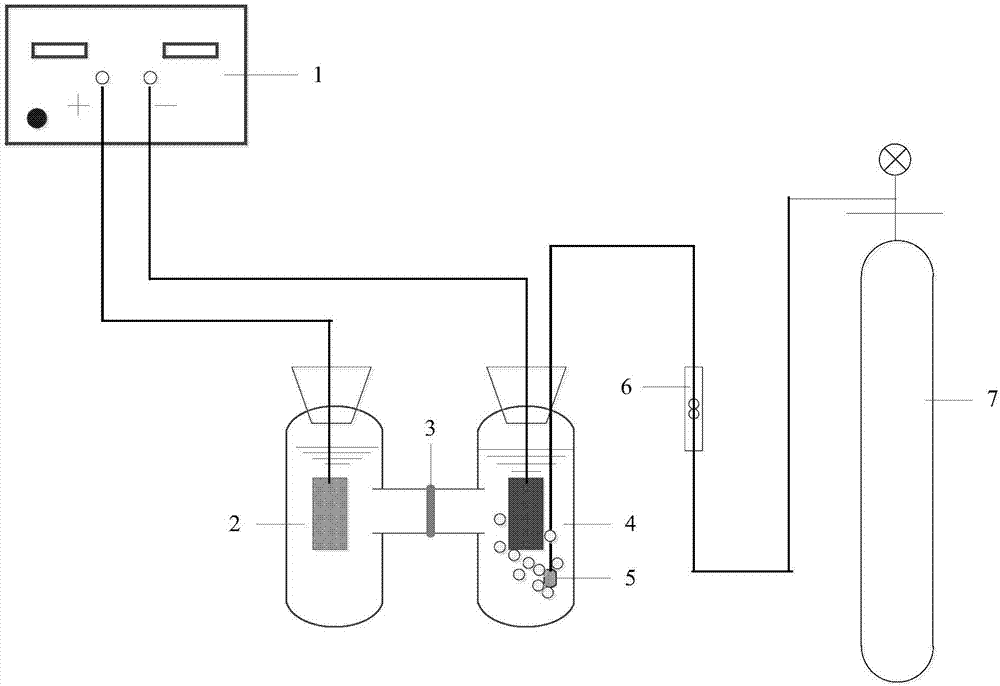
Preparation method of Fe-FeOx/carbon aerogel self-supporting cathode and heterogeneous electro-Fenton methyl orange degradation system
A carbon aerogel, self-supporting technology, used in chemical instruments and methods, water/sludge/sewage treatment, water/sewage treatment, etc., which can solve harsh reaction conditions, difficult to form substrate materials, and difficult to degrade azo dyes and other problems to achieve the effect of strengthening the degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
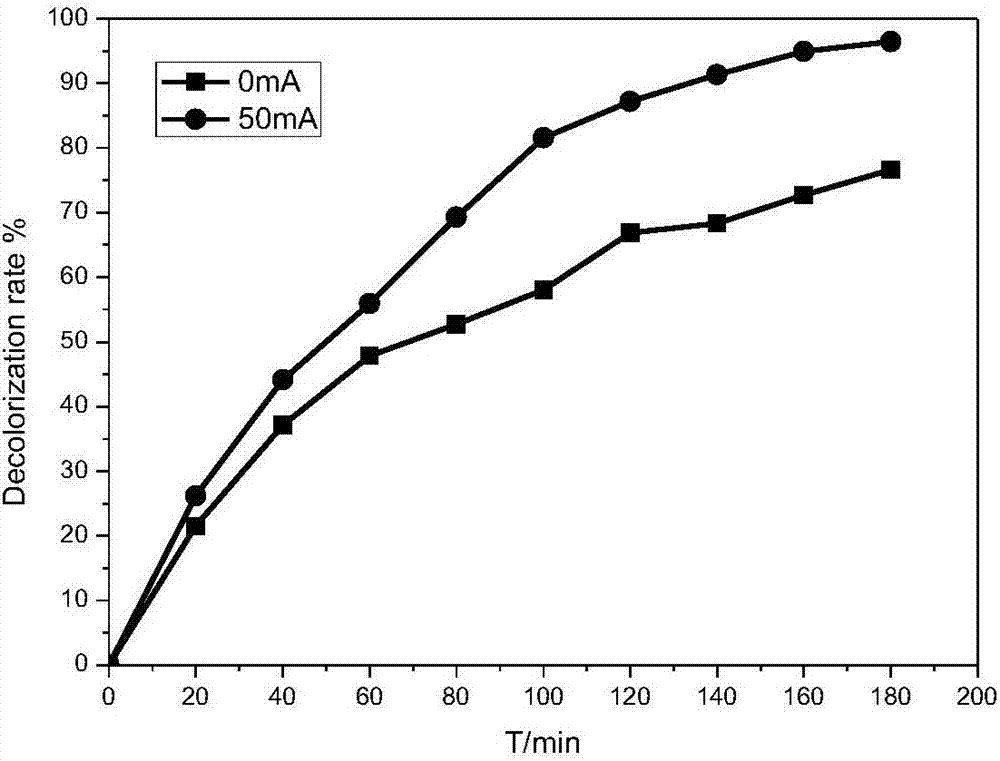
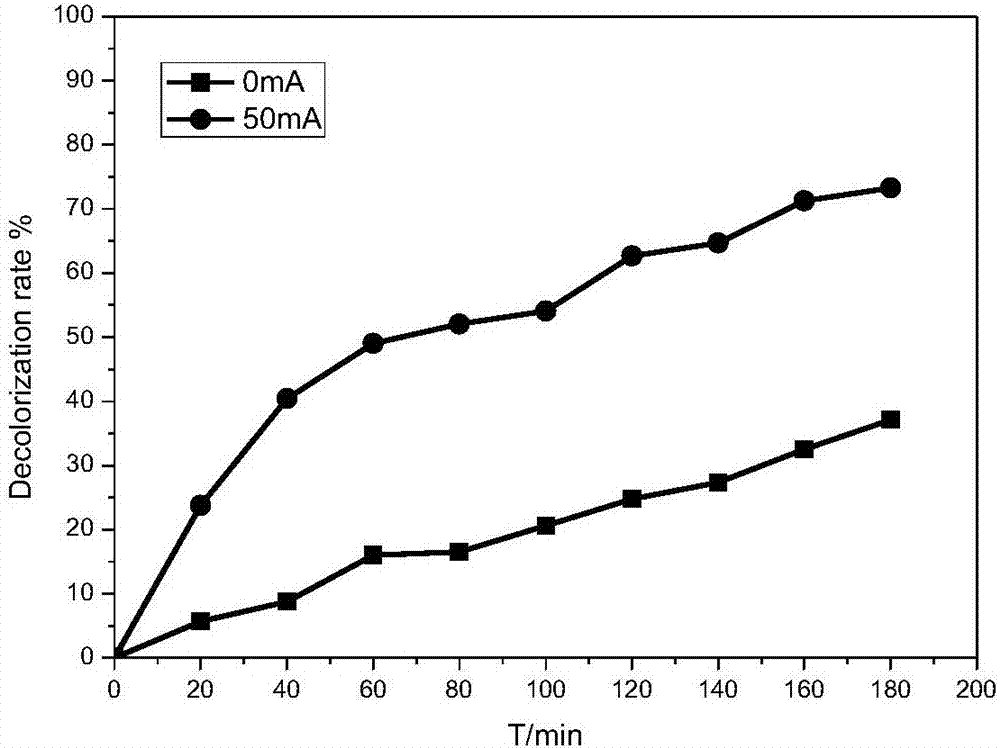
[0026] Example 1: Comparison of adsorption performance and electrochemical oxidation performance of different electrode materials (Fe loads are 0%, 0.5%, 5%) on methyl orange decolorization effect comparison test and different Fe loads on methyl orange decolorization effect impact testing.
[0027] Reaction conditions: prepare methyl orange simulated wastewater 150mg / L, add appropriate amount of Na 2 SO 4 To adjust the conductivity of the solution, add 60mL of the above solution to the cathode and anode chambers respectively, and use FCAs electrodes with Fe loads of 0%, 0.5% and 5% as cathodes. At pH=7, the aeration rate is 1.4L / min. Under the conditions of 0mA and 50mA, react for 180min, measure the concentration of methyl orange and calculate its decolorization rate.
[0028] The result is shown in Figure 2: Figure 2.1It is a cathode material with Fe loading of 0%. The results show that the decolorization effect of methyl orange is not very different between its adsorpti...
Embodiment 2
[0029] Example 2: Using an FCAs electrode with a Fe load of 5% as a cathode, the influence of current on the decolorization effect of methyl orange was investigated.
[0030] Reaction conditions: prepare methyl orange simulated wastewater 150mg / L, add appropriate amount of Na 2 SO 4 Adjust the conductivity of the solution, add 60mL of the above solution to the cathode and anode chambers, use the FCAs electrode with a Fe load of 5% as the cathode, and the aeration rate is 1.4L / min, pH=7, and the investigation current is 10mA, 30mA and 50mA Under the conditions of 180min, the decolorization rate of methyl orange.
[0031] The result is as image 3 Shown: With the increase of current, the decolorization rate of methyl orange is obviously enhanced, after 180min, under the condition of 10mA, 30mA and 50mA, the decolorization rate is 52.59%, 77.05% and 100% respectively.
Embodiment 3
[0032] Example 3: Using the FCAs electrode with a Fe load of 5% as the cathode, the influence of the amount of aeration on the decolorization effect of methyl orange was investigated.
[0033] Reaction conditions: prepare methyl orange simulated wastewater 150mg / L, add appropriate amount of Na 2 SO 4 Adjust the conductivity of the solution, add 60mL of the above solution to the cathode and anode chambers respectively, use the FCAs electrode with a Fe load of 5% as the cathode, and check the aeration rate at 0L / min and 0.7L / min at a current of 50mA and pH=7 And 1.4L / min conditions, reaction 180min, the decolorization rate of methyl orange.
[0034] The result is as Figure 4 Shown: With the increase of aeration, the decolorization rate of methyl orange is obviously enhanced. After 180min, under the conditions of 0L / min, 0.7L / min and 1.4L / min, the decolorization rate is 48.79%, 95.10% respectively and 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com