Organic-inorganic super-hydrophilic and underwater super-lipophobic fabric and preparation method and application thereof
An underwater super-oleophobic and super-hydrophilic technology, applied in the field of super-hydrophilic materials, can solve the problems of cumbersome preparation of super-hydrophilic fabrics, harsh reaction conditions, expensive instruments, etc., and achieve good recyclability and high separation efficiency , the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 2.46g tetraethyl orthosilicate, 0.20g (0.50mmol) trimethylolpropane tris(3‐mercaptopropionate) and 0.41g (0.75mmol) polyethylene glycol dimethacrylate with a molecular weight of 550 Make a solution in 3.07g ethanol, soak the polyester fabric in the solution for 1min, take it out and place it in a closed container containing 5g ammonia water and 5g n-butylamine without contacting ammonia water and n-butylamine, put the closed container in an oven And react at a temperature of 45°C for 1.5h to prepare organic-inorganic superhydrophilic and underwater superoleophobic fabrics.
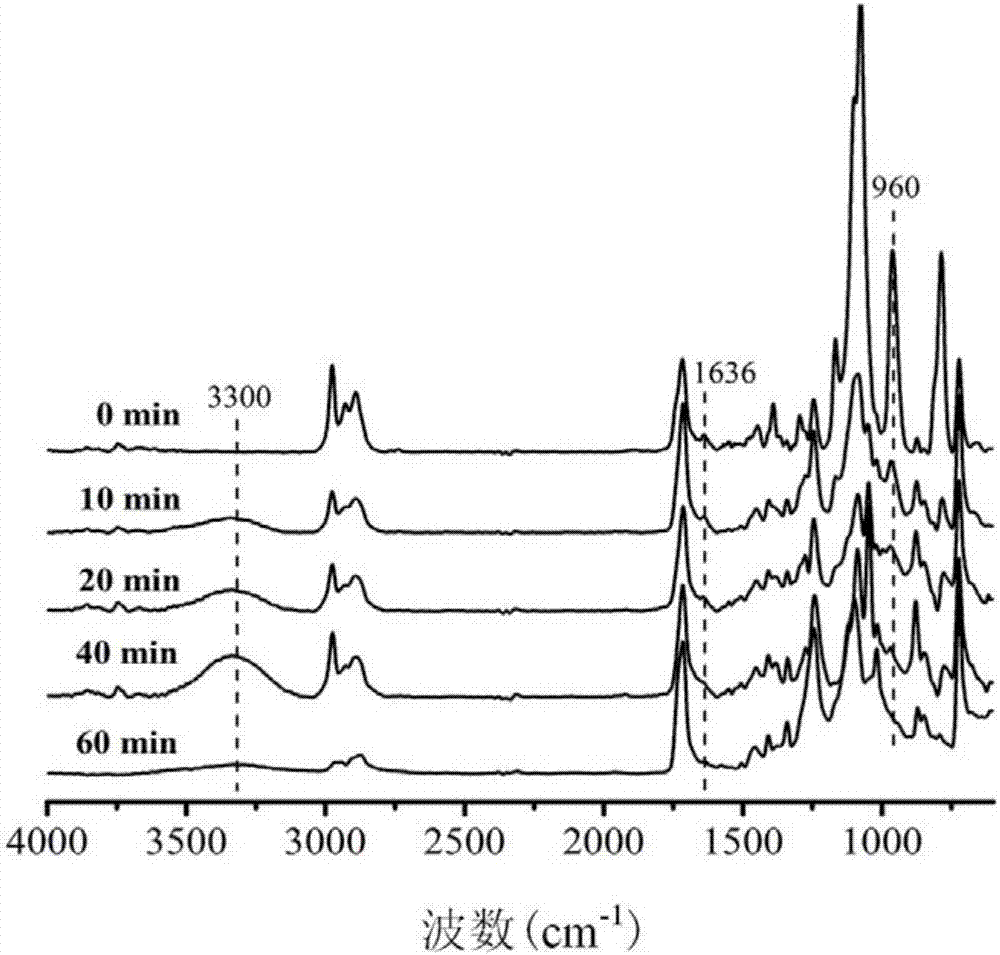
[0033] figure 1 It is the total reflection infrared spectrum measured after soaking the treated fabric under the catalysis of the mixed solution of ammonia water and n-butylamine respectively for 0 min, 10 min, 20 min, 40 min and 60 min. From figure 1 1636 cm corresponding to the C=C double bond can be observed in ‐1 The peak at gradually becomes smaller and finally disappears, which proves t...
Embodiment 2
[0040] Add 2.35g tetraethyl orthosilicate, 0.20g (0.50mmol) trimethylolpropane tris(3‐mercaptopropionate) and 4.5g (0.75mmol) polyethylene glycol dimethacrylate with a molecular weight of 6000 Make a solution in 7.05g ethanol, soak the cotton fabric for 5min, take it out and place it in a closed container with 5g ammonia water and 2.5g n-butylamine without contacting ammonia water and n-butylamine, put the closed container into an oven and After reacting at 30°C for 3 hours, organic and inorganic superhydrophilic and underwater superoleophobic fabrics can be prepared.
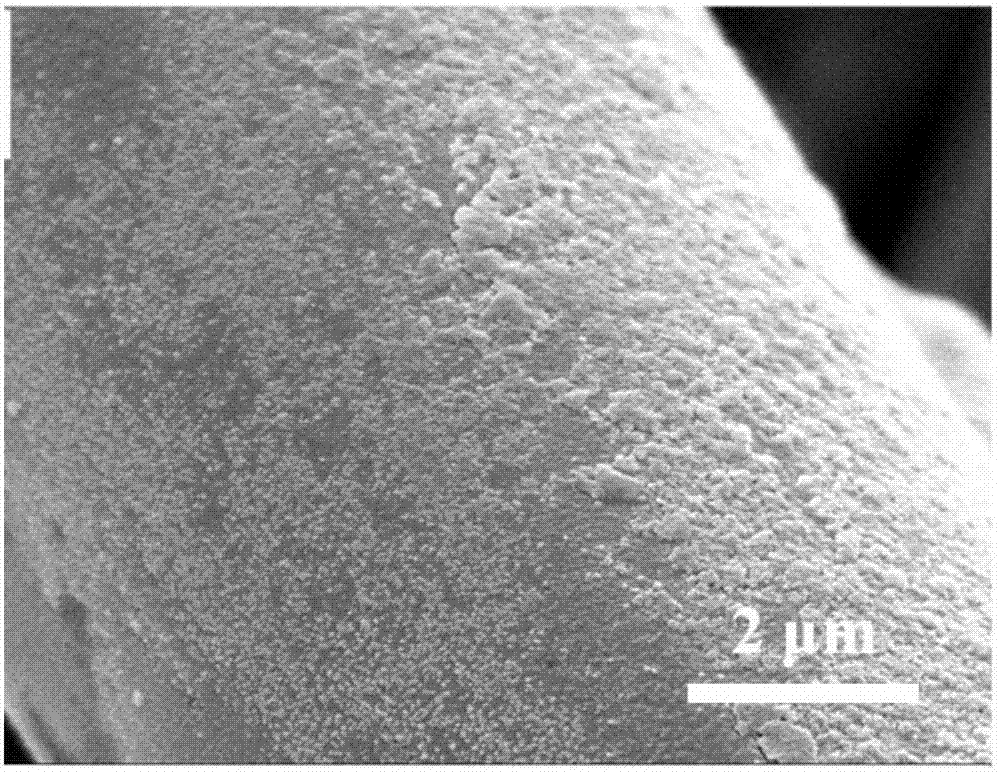
[0041] The scanning electron micrographs of organic and inorganic superhydrophilic and underwater superoleophobic fabrics of this embodiment and the process of water droplets reaching 0 ° contact angle are respectively compared with figure 1 and figure 2 Similarly, it shows that the surface of the fabric has a micro-nano rough structure and is super-hydrophilic.
[0042] Table 1 lists the contact angles of u...
Embodiment 3
[0044] Add 5.1g tetraethyl orthosilicate, 0.20g (0.50mmol) trimethylolpropane tris(3‐mercaptopropionate) and 1.5g (0.75mmol) polyethylene glycol dimethacrylate with a molecular weight of 2000 Make a solution in 6.8g ethanol, soak the nylon fabric for 3min, take it out and place it in a closed container with 7.5g ammonia water and 2.5g n-butylamine without contacting ammonia water and n-butylamine, put the closed container into an oven and After reacting at 60°C for 0.5h, organic and inorganic superhydrophilic and underwater superoleophobic fabrics can be prepared.
[0045] The scanning electron micrographs of organic and inorganic superhydrophilic and underwater superoleophobic fabrics of this embodiment and the process of water droplets reaching 0 ° contact angle are respectively compared with figure 1 and figure 2 Similarly, it shows that the surface of the fabric has a micro-nano rough structure and is super-hydrophilic.
[0046] Table 1 lists the contact angles of under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com