Long-circulating nanoparticle for targeting circulating tumor cells as well as preparation and application thereof
A technology of tumor cells and nanoparticles, which is applied in the field of long-circulation nanoparticles and its preparation and application, can solve the problems of inability to effectively utilize the specific recognition of polypeptides, and achieve improved drug bioavailability, good physiological compatibility, and encapsulation high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of DTX-SLNs-PepCD47
[0043] This embodiment provides the preparation method of SLNs as a control experiment, specifically as follows:
[0044]Weigh 3mg of DTX and 20mg of soybean lecithin into a vial, add 2mL of methanol to dissolve. Weighing 1 mg of SA-PepCD47 was dissolved in a small amount of dimethyl sulfoxide, and then mixed with the above methanol solution to obtain a mixed solution. At the same time, take 60 mg of glyceryl monostearate and melt it in a water bath at 70° C. to obtain a molten liquid. The above-mentioned mixed solution is mixed with the molten liquid to obtain a uniform oil phase, and then the water phase containing the emulsifier (preheated to 70°C) was quickly added to the oil phase, after dispersion, placed in an ultrasonic cell pulverizer for ultrasonication, stirred for 5 hours to evaporate the organic solvent, and DTX-SLNs-PepCD47 was obtained. Wherein, the emulsifier is Tween-80. The particle size, potential, drug l...
Embodiment 2
[0045] Example 2 Preparation of DTX-SLNs-Pep10
[0046] This embodiment provides the preparation method of SLNs as a control experiment, specifically as follows:
[0047] Weigh 3mg of DTX and 20mg of soybean lecithin into a vial, add 2mL of methanol to dissolve. Weighing 3 mg of SA-Pep10 was dissolved in a small amount of dimethyl sulfoxide, and then mixed with the above methanol solution to obtain a mixed solution. At the same time, take 60 mg of glyceryl monostearate and melt it in a water bath at 70° C. to obtain a molten liquid. The above-mentioned mixed solution is mixed with the molten liquid to obtain a uniform oil phase, and then the water phase containing the emulsifier (preheated to 70°C) was quickly added to the oil phase, after dispersion, placed in an ultrasonic cell pulverizer for ultrasonication, stirred for 5 hours to evaporate the organic solvent, and DTX-SLNs-Pep10 was obtained. Wherein, the emulsifier is Tween-80. The particle size, potential, drug loadin...
Embodiment 3
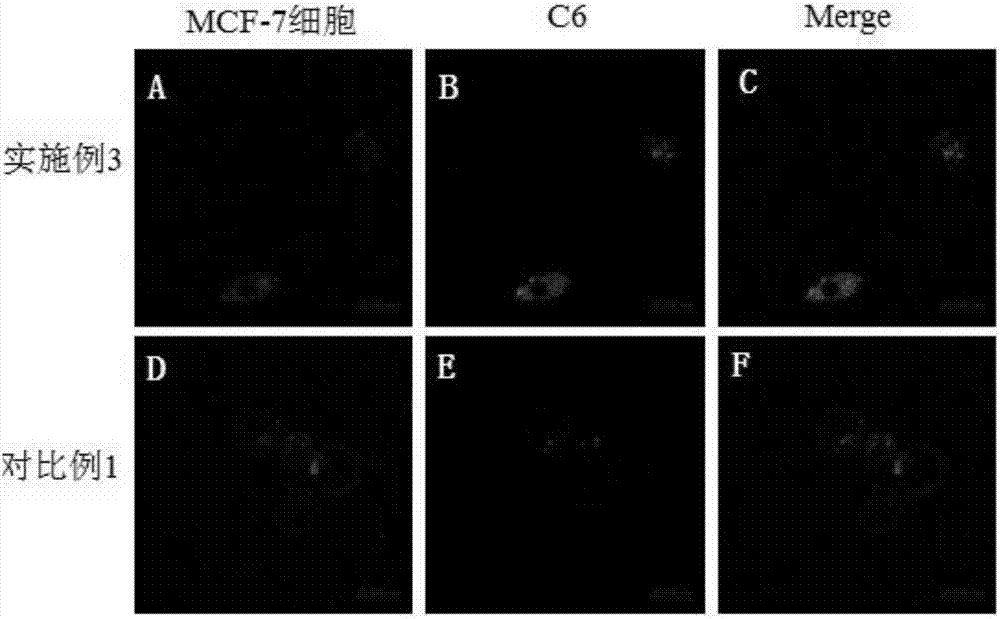
[0048] Example 3 Preparation of DTX-SLNs-PepCD47-Pep10
[0049] The preparation method of the long-circulating nanoparticles targeting circulating tumor cells of the present invention is as follows:
[0050] Weigh 3mg of DTX and 20mg of soybean lecithin into a vial, add 2mL of methanol to dissolve. 1 mg SA-PepCD47 and 3 mg SA-Pep10 were weighed and dissolved in a small amount of dimethyl sulfoxide, and then mixed with the above methanol solution to obtain a mixed solution. At the same time, 60 mg of glyceryl monostearate was weighed and melted in a water bath at 70° C. to obtain a molten liquid. The above-mentioned mixed solution is mixed with the molten liquid to obtain a uniform oil phase. The emulsifier was dispersed in purified water in a water bath at 70°C to prepare a water phase, wherein the emulsifier was Tween-80. Then, the aqueous phase (preheated to 70°C in advance) was quickly added to the oil phase, after dispersion, placed in an ultrasonic cell pulverizer for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com