A kind of fluorine-containing double active group orange dye and preparation method thereof
A dual-reactive group and orange technology, applied in the direction of reactive dyes, organic dyes, azo dyes, etc., to achieve the effect of less hydrolyzed dyes, high dyeing rate and color fixation rate, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
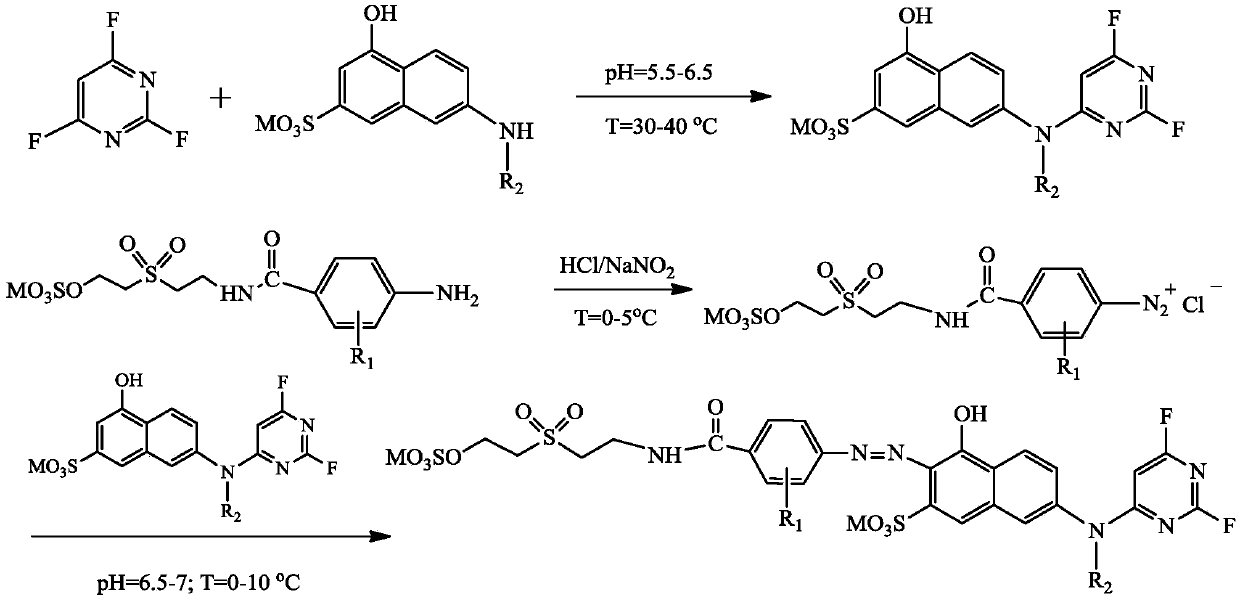
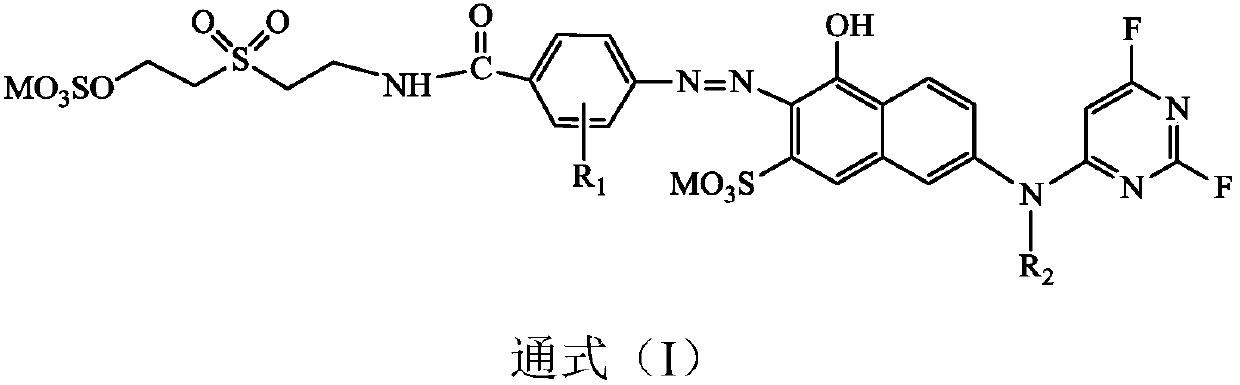
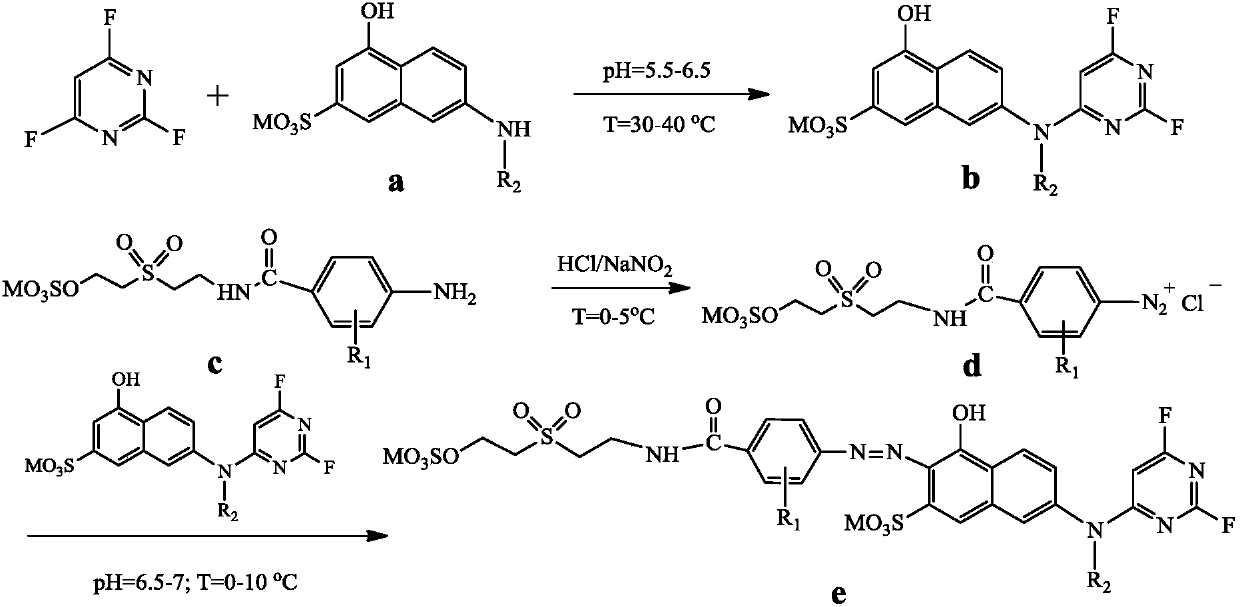
[0027] A kind of fluorine-containing double reactive group orange dye A, its structural formula is as follows:
[0028]
[0029] The preparation method of described orange reactive dye A comprises the steps:
[0030] (1) Add 23.9g of 100% J acid into 400mL water, add 20% NaOH solution under stirring, adjust the pH value to 6.5, dissolve it, raise the temperature to 30°C, add dropwise 13.94g (99%) 2,4 , 6-trifluoropyrimidine, control the pH value at 5.5 and continue the reaction for 2 hours, the Ehrlich reagent detects the end point of the reaction, and the reaction solution is ready for use after the condensation is completed;
[0031] (2) Add 35.2 g of 100% 4-[2-[2-(sulfonic acid oxyethylsulfone) ethyl]-carbamoyl]-aniline into an appropriate amount of ice for beating for 1 hour, and add 12.8 g of industrial hydrochloric acid (30%), continue to stir and cool to between 5 DEG C, dropwise 23g sodium nitrite solution (30% mass concentration) in the above-mentioned beating liq...
Embodiment 2
[0034] A kind of fluorine-containing double reactive group orange dye B, its structural formula is as follows:
[0035]
[0036] The difference from the above-mentioned Example 1 is that in this example, 4-[2-[2-(sulfonic acid oxyethylsulfone) ethyl]-carbamoyl]-aniline-2-sulfonic acid is used instead of 4 -[2-[2-(sulfonic acid oxyethyl sulfone) ethyl]-carbamoyl]-aniline to prepare the corresponding diazonium salt, and then the condensation product of 2,4,6-trifluoropyrimidine and J acid The orange reactive dye B was obtained by coupling.
Embodiment 3
[0038] A kind of fluorine-containing double active group orange dye C, its structural formula is as follows:
[0039]
[0040] The difference from the above-mentioned Example 2 is that in this example, 4-[2-[2-(sulfonic acid oxyethylsulfone) ethyl]-carbamoyl]-2-methoxyaniline is used instead of 4 -[2-[2-(sulfonic acid oxyethyl sulfone) ethyl]-carbamoyl]-aniline to prepare the corresponding diazonium salt, and then the condensation product of 2,4,6-trifluoropyrimidine and J acid The orange reactive dye C was obtained by coupling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com