Preparation method of inflammation-response guided tissue regeneration membrane
An inflammation and initiator technology, applied in the field of preparation of inflammatory response type releasing anti-inflammatory drug film, can solve the problem of inability to prevent and suppress individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
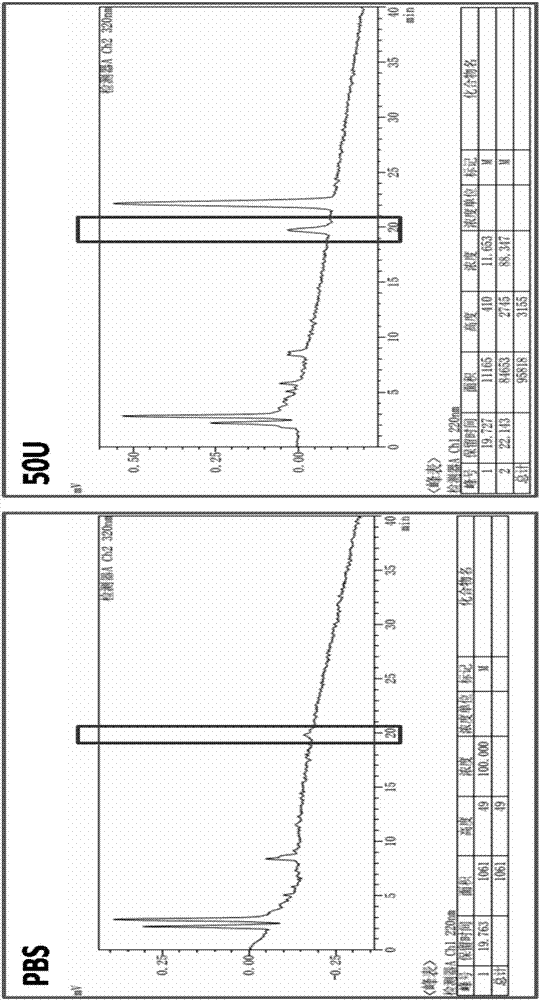
Image
Examples
Embodiment 1
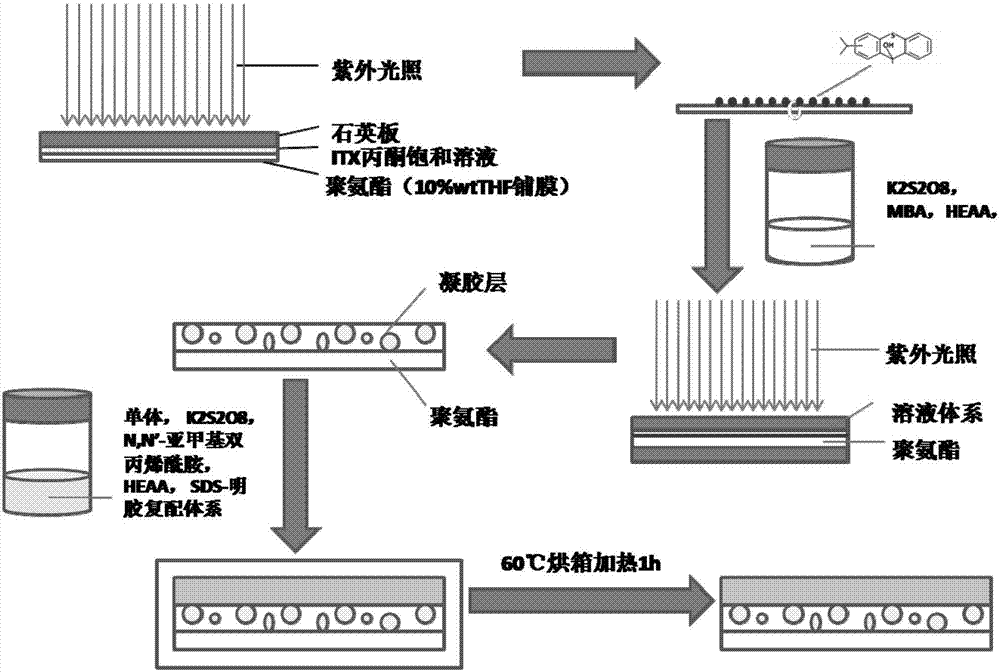
[0038] (1) On the surface of electrospun porous polyurethane (PU), apply a saturated solution of ITX acetone on the surface of polyurethane, cover the surface of polyurethane, cover with a quartz plate, and irradiate with ultraviolet light for 10 minutes (wavelength 200nm, irradiation distance 50mm ), the ITXSP functional group was introduced into the surface of polyurethane (PU) membrane (thickness 100 μm, average pore size 1 μm).
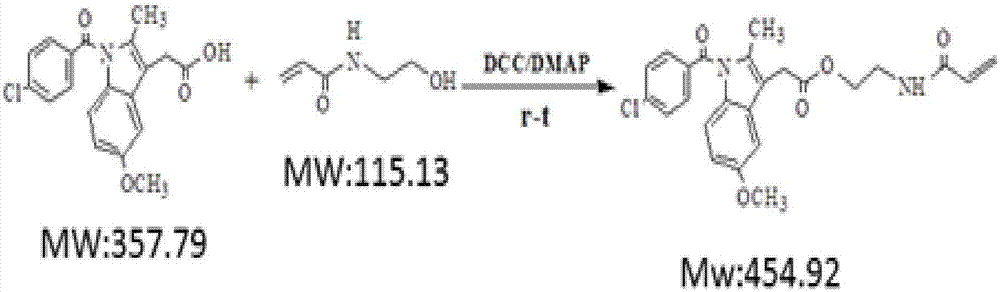
[0039] (2) Carried out by N-hydroxyethylacrylamide (HEMAA) and indomethacin (IDCM) under the catalysis of DCC / DMAP (N, N'-dicyclohexylcarboimide / 4-dimethylaminopyridine) Esterification reaction to obtain monomers containing drugs, ester bonds and double bonds. The molar ratio of the reaction monomer to the initiator is IDCM:HEMAA:DCC:DMAP=1:3:3:0.5, the solvent is anhydrous DMF, the reaction temperature is 40°C, and the reaction time is 24h. First add other raw materials except DCC at one time, then add DCC under ice bath conditions, and continue...
Embodiment 2
[0043] (1) On the surface of electrospun porous polyurethane (PU), apply a saturated solution of ITX acetone on the surface of polyurethane, cover the surface of polyurethane, cover with a quartz plate, and irradiate with ultraviolet light for 10 minutes (wavelength 400nm, irradiation distance 50mm ), introducing ITXSP functional groups to the surface of polyurethane (PU) membrane (thickness 1mm, average pore size 10 μm);
[0044] (2) Carried out by N-hydroxyethylacrylamide (HEMAA) and indomethacin (IDCM) under the catalysis of DCC / DMAP (N, N'-dicyclohexylcarboimide / 4-dimethylaminopyridine) Esterification reaction to obtain monomers containing drugs, ester bonds and double bonds. The molar ratio of the reaction monomer to the initiator is IDCM:HEMAA:DCC:DMAP=1:3:3:0.5, the solvent is anhydrous DMF, the reaction temperature is 40°C, and the reaction time is 24h. First add other raw materials except DCC at one time, then add DCC under ice bath conditions, and continue the ice b...
Embodiment 3
[0048] (1) On the surface of electrospun porous polyurethane (PU), apply a saturated solution of ITX acetone on the surface of polyurethane, cover the surface of polyurethane, cover with a quartz plate, and irradiate with ultraviolet light for 10 minutes (wavelength 350nm, irradiation distance 50mm ), introducing ITXSP functional groups to the surface of polyurethane (PU) membrane (thickness 0.5mm, average pore size 6μm);
[0049] (2) Carried out by N-hydroxyethylacrylamide (HEMAA) and indomethacin (IDCM) under the catalysis of DCC / DMAP (N, N'-dicyclohexylcarboimide / 4-dimethylaminopyridine) Esterification reaction to obtain monomers containing drugs, ester bonds and double bonds. The molar ratio of the reaction monomer to the initiator is IDCM:HEMAA:DCC:DMAP=1:3:3:0.5, the solvent is anhydrous DMF, the reaction temperature is 40°C, and the reaction time is 24h. First add other raw materials except DCC at one time, then add DCC under ice bath conditions, and continue the ice b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com