Preparation method of calcium sulfite particles having amorphous structure
A calcium sulfite, amorphous technology, applied in the direction of calcium/strontium/barium sulfite, etc., to achieve good turbidity removal effect, environmental protection of raw materials, and uniform distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
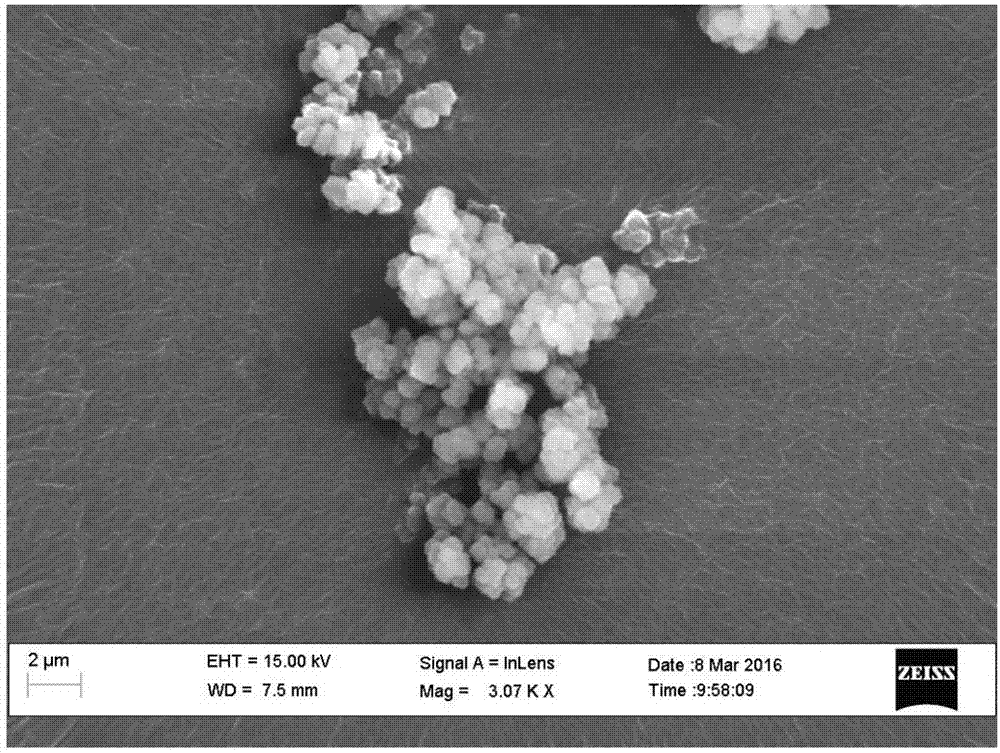
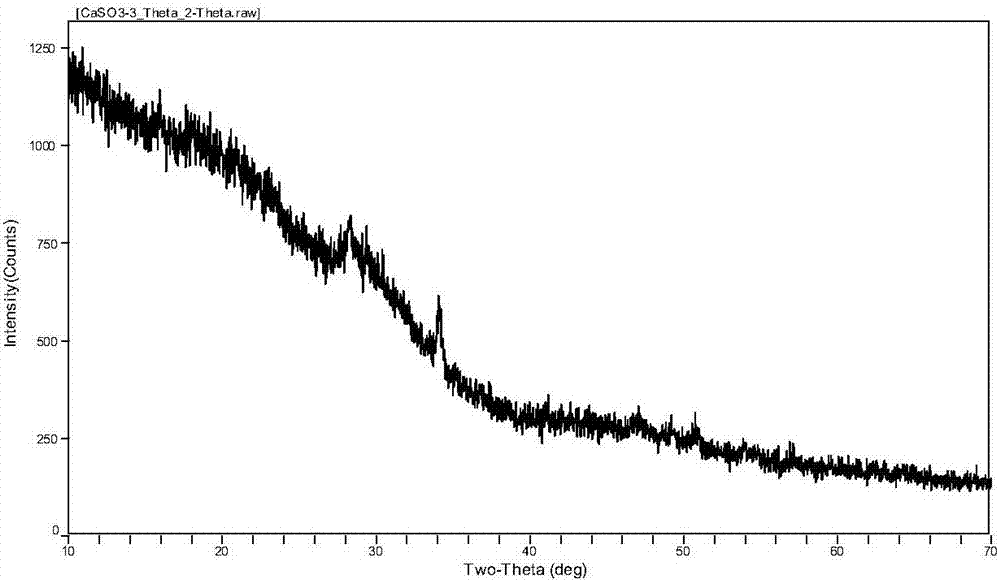
[0017] Example 1: In a 200ml beaker, prepare a milk of lime solution with a mass fraction of 5% and a sodium hexametaphosphate solution of 1g / L with water respectively in a 200ml beaker, then filter the sodium hexametaphosphate solution with a 0.45 μm filter membrane to remove undissolved Impurities, then take 20mL of sodium hexametaphosphate solution in the prepared 20g of lime milk solution, and continue to stir for 30min to fully react. Then add sulfurous acid into the reacted solution, adjust pH=7.0, and stir slowly to generate calcium sulfite. Finally, the generated calcium sulfite precipitate was filtered, centrifuged, washed alternately with double distilled water and absolute ethanol three times, and fully dried at 60°C. After crystal structure detection by XRD (X-ray diffraction) and morphology observation by SEM (scanning electron microscope), calcium sulfite with particle size distribution of 600-800nm, uniform distribution, amorphous structure and granular shape wa...
Embodiment 2
[0018] Example 2: In a 200ml beaker, the milk of lime solution of 5% and the sodium hexametaphosphate solution of 2g / L were prepared with water respectively in a 200ml beaker, and then the sodium hexametaphosphate solution was filtered with a 0.45 μm filter membrane to remove undissolved Impurities, then take 30mL of sodium hexametaphosphate solution in the prepared 48g of milk of lime solution, and continue to stir for 30min to fully react. Then add sulfurous acid into the reacted solution, adjust pH=7.0, and stir slowly to generate calcium sulfite. Finally, the generated calcium sulfite precipitate was filtered, centrifuged, washed alternately with double distilled water and absolute ethanol three times, and fully dried at 60°C. After crystal structure detection by XRD (X-ray diffraction) and morphology observation by SEM (scanning electron microscope), calcium sulfite with particle size distribution of 600-800nm, uniform distribution, amorphous structure and granular shape ...
Embodiment 3
[0019] Example 3: In a 200ml beaker, the milk of lime solution with a mass fraction of 10% and the sodium hexametaphosphate solution of 3g / L were prepared with water respectively in a 200ml beaker, and then the sodium hexametaphosphate solution was filtered with a 0.45 μm filter membrane to remove undissolved Impurities, then take 40mL of sodium hexametaphosphate solution in the prepared 80g of milk of lime solution, and continue to stir for 30min to fully react. Then add sulfurous acid into the reacted solution, adjust pH=7.0, and stir slowly to generate calcium sulfite. Finally, the generated calcium sulfite precipitate was filtered, centrifuged, washed alternately with double distilled water and absolute ethanol three times, and fully dried at 60°C. After crystal structure detection by XRD (X-ray diffraction) and morphology observation by SEM (scanning electron microscope), calcium sulfite with particle size distribution of 600-800nm, uniform distribution, amorphous structu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com