Catalyst for hydrogenation synthesis of methanol by using carbon dioxide and preparation and application thereof
A technology for synthesizing methanol from carbon dioxide, which is applied in the preparation of organic compounds, catalyst activation/preparation, and preparation of hydroxyl compounds. It can solve the problems of catalyst particle sintering, reduced catalyst activity, and low selectivity of methanol, and achieves mild and high preparation conditions. The effect of methanol synthesis activity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
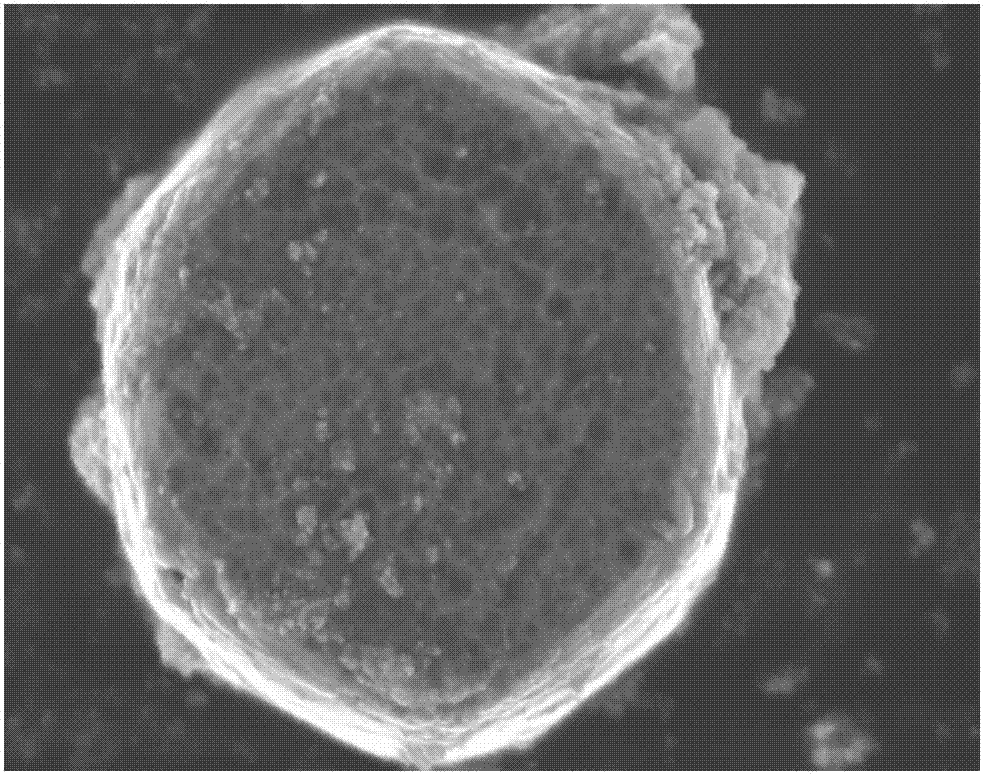
[0031] (1) 96mmol Zn (carbon dioxide CH 3 ) 2 2H 2 O was dissolved in 240 mL of deionized water, and 96 mmol of diethylenetriamine was added with continuous stirring. After dissolving, the solution was transferred to a 500 mL polytetrafluoroethylene-lined autoclave, kept at 100 °C for 3 h, and then slowly cooled to room temperature. The white precipitate was washed with deionized water, and finally dried at 60 °C for 12 h to obtain cylindrical ZnO, as figure 2 shown.
[0032] (2) 20mmol of Cu (carbon dioxide CH 3 ) 2 ·H 2 O was dissolved in 400 mL of deionized water, 10 mmol of cylindrical ZnO was added under continuous stirring, and the temperature of the solution was raised to 80 °C. Under continuous stirring, slowly drop 40 mL of L-ascorbic acid solution with a concentration of 3 mol / L to reduce copper ions. After the dropwise addition, keep warm for 5 hours, filter with suction, wash, and dry in vacuum at 60° C. for 12 hours. The catalyst is marked as Cu / cylindric...
Embodiment 2
[0034] The same operation as in Example 1 was used to prepare the catalyst, except that 192mmol Zn (carbon dioxide CH 3 ) 22H 2 O was dissolved in 240 mL of deionized water, and 192 mmol of hexamethylenetetramine was added under continuous stirring. The catalyst was labeled as Cu / filamentous ZnO catalyst, and filamentous ZnO as image 3 shown.
Embodiment 3
[0036] (1) Dissolve 24mmol of zinc nitrate in 240mL of deionized water, and add 24mmol of hexamethylenetetramine under continuous stirring. After dissolving, the solution was transferred to a 500 mL polytetrafluoroethylene-lined autoclave, kept at 150 °C for 6 h, and then slowly cooled to room temperature. The white precipitate was washed with deionized water, and finally dried at 60 °C for 12 h to obtain cylindrical ZnO.
[0037] (2) Dissolve 40 mmol of copper nitrate in 100 mL of deionized water, add 10 mmol of cylindrical ZnO under continuous stirring, and heat the solution to 40°C. Under continuous stirring, slowly dropwise add 80 mL of ethylene glycol solution with a concentration of 0.5 mol / L to reduce copper ions. After the dropwise addition, keep warm for 1 hour, filter with suction, wash, and dry in vacuum at 60° C. for 12 hours. The catalyst is marked as Cu / cylindrical ZnO.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com