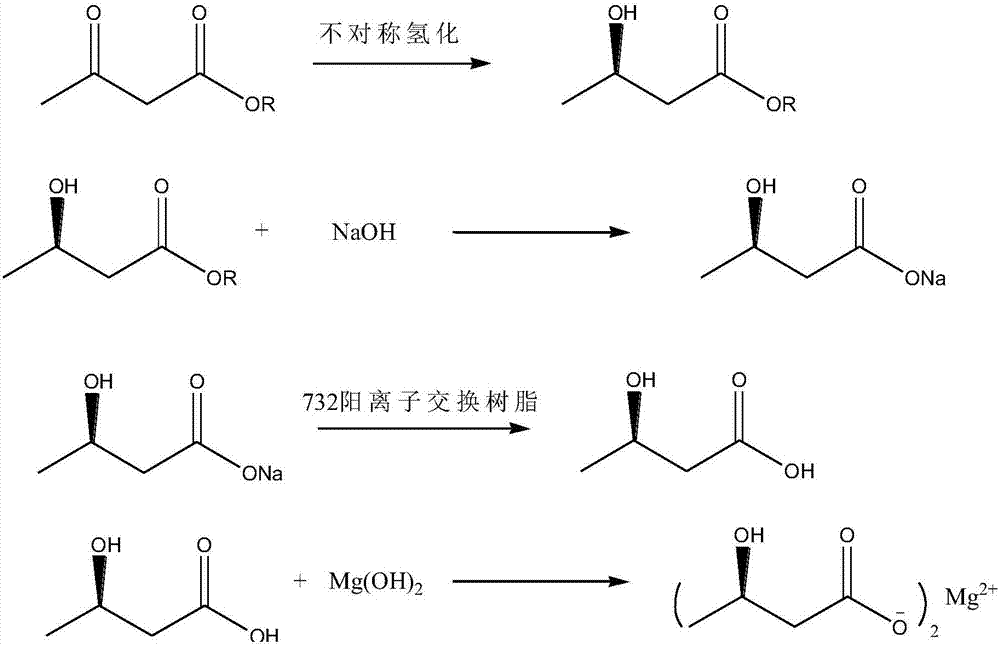
Synthesis process of (R)-3-hydroxybutyric acid and salts thereof
A technology of hydroxybutyric acid and hydroxybutyrate, applied in the synthesis process of magnesium salt, calcium salt, potassium salt, -3-hydroxybutyric acid and its sodium salt field, can solve the problem of large production input and enantiomeric excess value. Low cost, complex process and other problems, to achieve the effect of convenient post-processing, low cost and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: synthetic (R)-3-hydroxybutyric acid methyl ester
[0037] Dichlorophenylruthenium(II) dimer [RuCl 2 (benzene) 2 ](80mg, 160umol), (R)-(+)-2,2'-bis(diphenylphosphino)-1,1'-dibenzofuranyl-8,8'-dipotassium disulfonate Salt ligand (340umol) and methyl 3-oxobutanoate (74ml, 680mmol) were dissolved in methanol (600ml). After degassing under reduced pressure and purging the autoclave with nitrogen, the hydrogenation reaction was carried out at 60° C. and a hydrogen pressure of 10 bar for 16 hours. Cool to room temperature and concentrate to near dryness to obtain a mixture of catalyst and methyl (R)-3-hydroxybutyrate. Slowly heat up to 80°C and distill under reduced pressure to obtain colorless methyl (R)-3-hydroxybutyrate (76.0 g) with a yield of 94.7% and an ee value of 94%. The catalyst is recovered and can be reused after regeneration. The concentrated and recovered methanol can be directly reused.
[0038] MS:119(M+H)
Embodiment 2
[0039] Embodiment 2: synthetic (R)-3-hydroxybutyrate ethyl ester
[0040] Dichlorophenylruthenium(II) dimer [RuCl 2 (benzene) 2 ](80mg, 160umol), (R)-(+)-2,2'-bis(diphenylphosphino)-1,1'-dibenzofuranyl-8,8'-dipotassium disulfonate Salt ligand (340umol) and ethyl 3-oxobutanoate (86ml, 680mmol) were dissolved in methanol (600ml). After degassing under reduced pressure and purging the autoclave with nitrogen, the hydrogenation reaction was carried out at 80° C. and a hydrogen pressure of 10 bar for 20 hours. Cool to room temperature and concentrate to near dryness to obtain a mixture of catalyst and ethyl (R)-3-hydroxybutyrate; slowly warm up to 85°C and distill under reduced pressure to obtain colorless ethyl (R)-3-hydroxybutyrate 88.4 g, yield 98.5%, ee value 94.5%. The catalyst is recovered and can be reused after regeneration. The methanol recovered by concentration can be used directly.
[0041] MS:133(M+H)
Embodiment 3
[0042] Embodiment 3: preparation (R)-3-hydroxybutyrate sodium
[0043] In a 500ml three-necked flask, add the (R)-3-hydroxybutyrate methyl ester (76.0g) obtained in Example 1, add 300ml of water, control the temperature to less than 30°C, and slowly add the hydroxide in batches within 3 hours Sodium (25.8g), control the temperature not higher than 10°C, continue to react for 3 hours until the reaction is complete. Add 0.3 g of activated carbon, continue to stir for 0.5 hours, filter, and concentrate the filtrate to 100 ml. At this time, a large amount of solids precipitate out, cool down to room temperature, stir and crystallize for 1 hour, centrifuge, and dry in vacuo to obtain white solid (R)-3-hydroxybutyrate Sodium acid 68.9g, yield 85.0%, ee value 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com