A drug targeted delivery carrier, preparation method and application thereof
A carrier and drug technology, applied in the direction of pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve the problems of non-targeted distribution, easy to be degraded during transportation, etc., and achieve stable shape and structure, complete protection, and good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
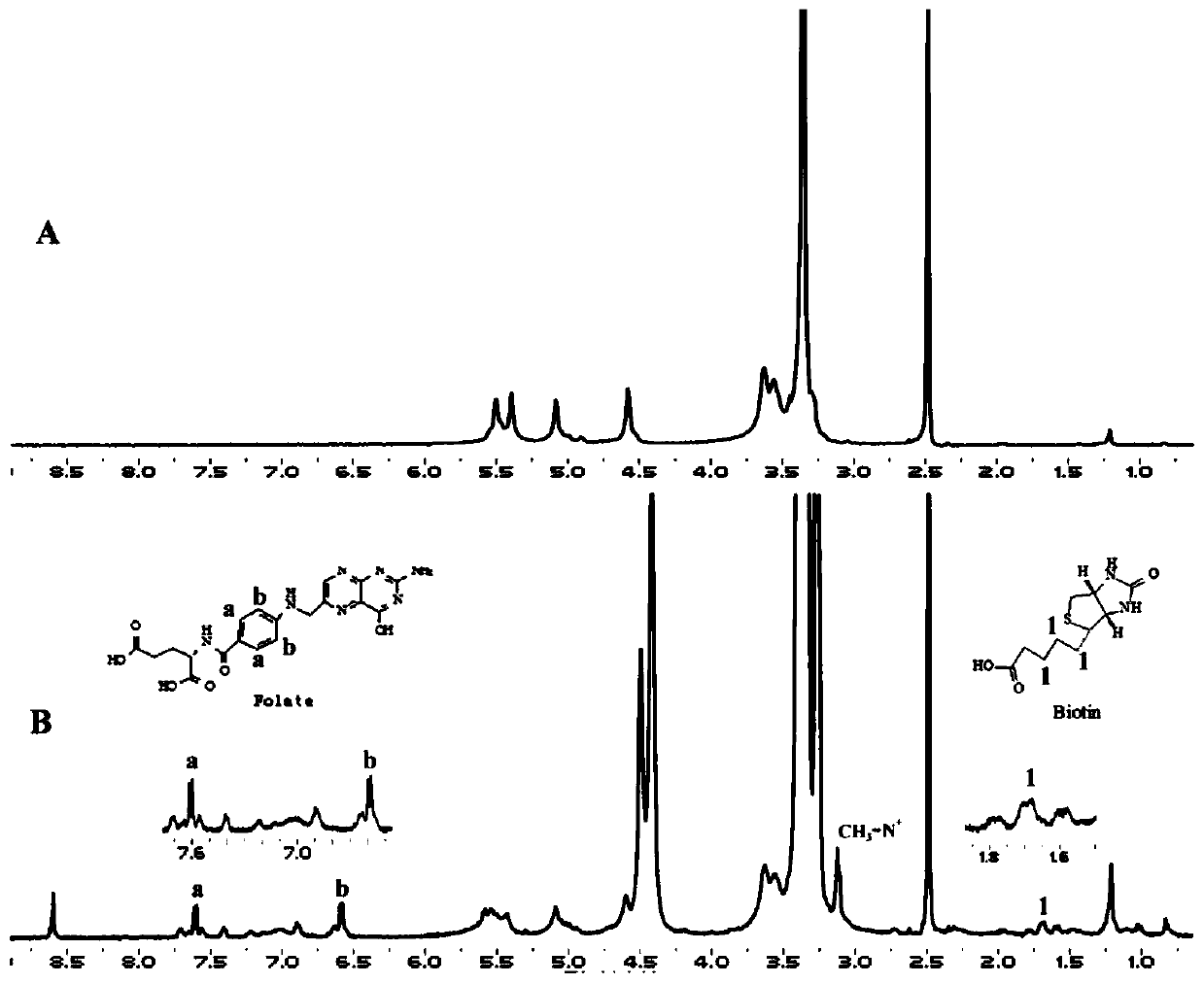
Image
Examples
Embodiment 1
[0042] Preparation of folic acid-biotin-quaternized starch nanoparticles
[0043] The experimental principle is as follows,
[0044]
[0045] The specific preparation method is as follows:
[0046] a) preparation steps of quaternized starch:
[0047] 20 grams of potato starch is fully soaked in 12ml of isopropanol to form a starch isopropanol solution;
[0048] Dissolve 5.925 grams of NaOH in 8.24 grams of deionized water to form a NaOH solution, then pour 38.7 grams of 60% WT. etherification agent CTA into the NaOH solution, and stir at a high speed for 15 minutes to generate 2,3-epoxypropyl three Methyl ammonium chloride (GTA) solution;
[0049] Pour the generated 2,3-epoxypropyltrimethylammonium chloride (GTA) into the starch isopropanol solution at room temperature and stir at high speed for 1 hour to obtain a mixture, which is sealed and placed at 65°C React for 4 hours, cool to room temperature to generate white quaternized starch primary product, add anhydrous acet...
Embodiment 2
[0062] Preparation of folic acid-biotin-quaternized starch nanoparticles
[0063] The specific preparation method is as follows:
[0064] a) preparation steps of quaternized starch:
[0065] 20 grams of natural potato starch is fully soaked in 16ml of isopropanol to form a starch isopropanol solution;
[0066] Dissolve 5.925 grams of NaOH in 8.24 grams of deionized water to form a NaOH solution, then pour 38.7 grams of 60% WT. etherification agent CTA into the NaOH solution, and stir at a high speed for 15 minutes to generate 2,3-epoxypropyl three Methyl ammonium chloride (GTA) solution;
[0067] Pour the generated 2,3-epoxypropyltrimethylammonium chloride (GTA) into the starch isopropanol solution at room temperature and stir at high speed for 1 hour to obtain a mixture, which is sealed and placed at 65°C React for 4 hours, cool to room temperature to generate white quaternized starch primary product, add anhydrous acetic acid to neutralize to pH 6-8, then wash the quatern...
Embodiment 3
[0074] Preparation of folic acid-biotin-quaternized starch nanoparticles
[0075] The specific preparation method is as follows:
[0076] a) preparation steps of quaternized starch:
[0077] 20 grams of natural potato starch is fully soaked in 20ml of isopropanol to form a starch isopropanol solution;
[0078] Dissolve 5.925 grams of NaOH in 8.24 grams of deionized water to form a NaOH solution, then pour 38.7 grams of 60% WT. etherification agent CTA into the NaOH solution, and stir at a high speed for 15 minutes to generate 2,3-epoxypropyl three Methyl ammonium chloride (GTA) solution;
[0079] Pour the generated 2,3-epoxypropyltrimethylammonium chloride (GTA) into the starch isopropanol solution at room temperature and stir at high speed for 1 hour to obtain a mixture, which is sealed and placed at 65°C React for 4 hours, cool to room temperature to generate white quaternized starch primary product, add anhydrous acetic acid to neutralize to pH 6-8, then wash the quatern...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com