Callicarpa kwangtungensis extract separation preparation method and application of callicarpa kwangtungensis extract
A technology of extracts and purple beads, applied in medical preparations containing active ingredients, plant/algae/fungus/moss components, pharmaceutical formulas, etc., can solve the problems of immune inflammation such as UC that have not been found, and achieve strong promotion and application The effect of value, mild conditions, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] Example 1. Separation and preparation of the anti-ulcerative colitis drug of the present invention.
[0035] Separation and preparation method of effective groups with anti-ulcerative colitis activity in Guangdong Zizhu dry extract:
[0036] Preparation of Guangdong Zizhu dry extract: Take Guangdong Zizhu, moisten it thoroughly, cut into small pieces or slices, add water to decoct twice, the first 3 hours, the second 2 hours, filter, combine the filtrates, and concentrate the filtrates to Thick paste, dried under reduced pressure.
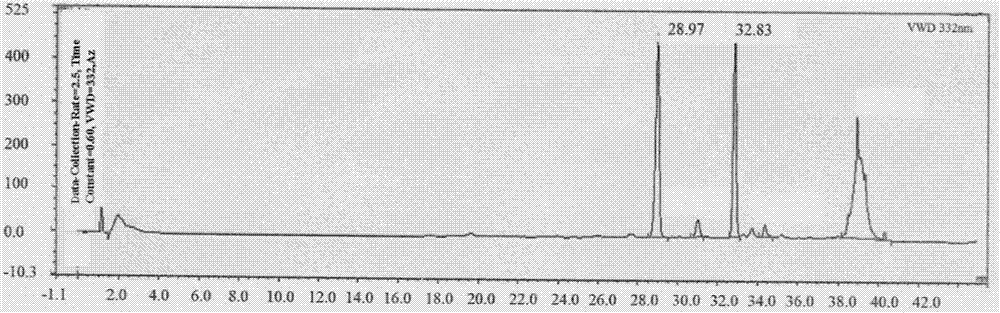
[0037]The Guangdong Zizhu dry extract was dissolved in water, the weight ratio of material to liquid was 1:40, and the supernatant was obtained by centrifugation at 5000r / min for 10min; passed through a 0.2um microfiltration system; the obtained permeate was passed through a 100k Da ultrafiltration membrane, pressure is 0.05MPa, ultrafiltered until the cut-off volume is 1 / 5 of the total volume of the original solution, and the same volume o...
example 2
[0038] Example 2. Separation and preparation of the anti-ulcerative colitis drug of the present invention.
[0039] The preparation method of active and effective group of anti-ulcerative colitis in the traditional Chinese medicine compound:
[0040] Preparation of Guangdong Zizhu dry extract: Take Guangdong Zizhu, moisten it thoroughly, cut into small pieces or slices, add water to decoct twice, the first 3 hours, the second 2 hours, filter, combine the filtrates, and concentrate the filtrates to Thick paste, dried under reduced pressure, and obtained. Preparation of motherwort dry extract: take motherwort, chop into pieces, add water to decoct twice for 2 hours each time, filter, combine the filtrates, concentrate the filtrate to a thick paste, and dry under reduced pressure. Preparation of dried black medicine extract: take black medicine, add water to decoct twice for 2 hours each time, filter, combine the filtrates, concentrate the filtrate into a thick paste, and dry un...
example 3
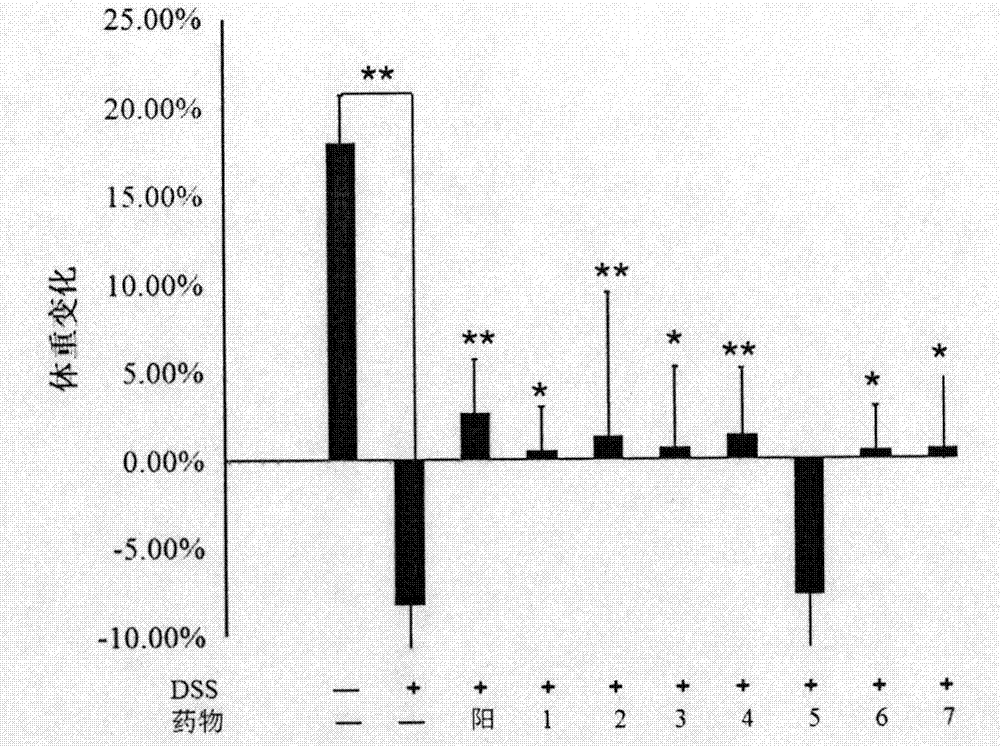
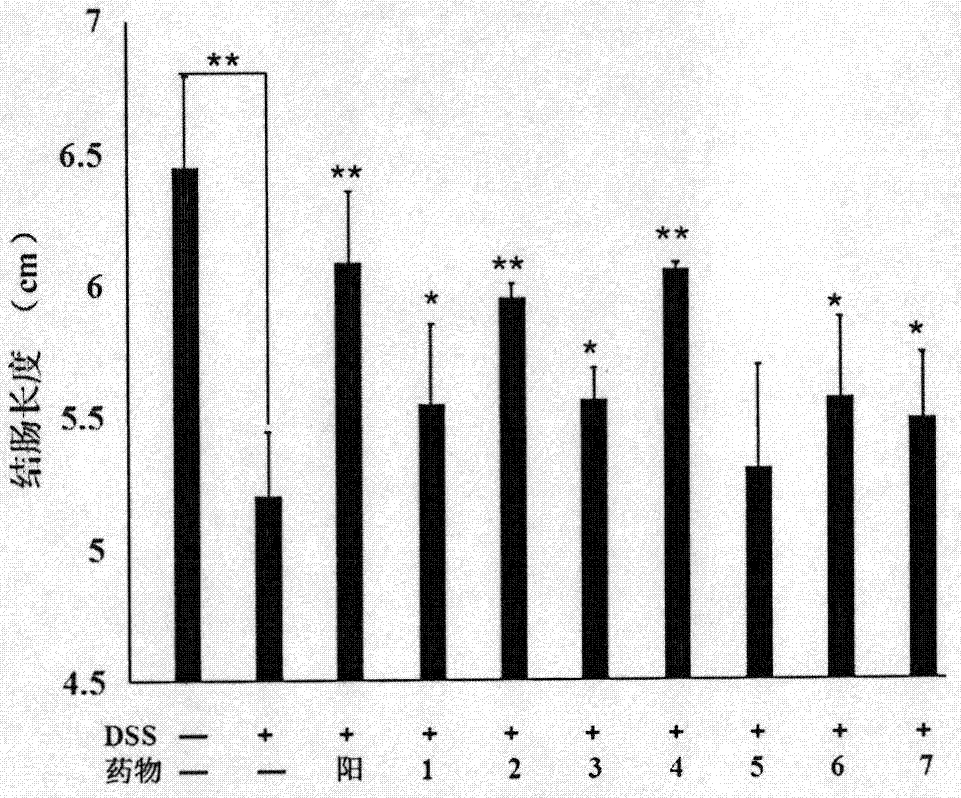
[0042] Example 3. Effect of the anti-ulcerative colitis drug of the present invention on mouse UC model
[0043] 1. Test materials
[0044] 1.1 Drugs and Dosages
[0045] The experimental drugs include 7 test drugs and sulfasalazine as the positive control drug, the specific dosage is shown in Table 1
[0046] Table 1. Tested Drugs and Dosages
[0047]
[0048] Test drug No. 1: the raw material used for the separation and preparation in Example 1, namely Guangdong Zizhu dry extract, wherein the Guangdong Zizhu polysaccharide content is 25.21%, and the phenylethanoid content is 6.3%. The test drug is the raw material prepared by separation.
[0049] Test drug No. 2: The final product obtained by separating and preparing the test drug No. 1 as the raw material according to the method described in Example 1, wherein the content of Guangdong Zizhu polysaccharide is 64.52%, and the content of phenethyl glucoside is 15.84%.
[0050] Test drug No. 3: the raw material used for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com