Preparation method and application of porous graphite
A technology of porous graphene and straw, applied in graphene, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of graphene electrode materials that are difficult to meet large-scale production, complex preparation process, high cost, etc., to increase added value , simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
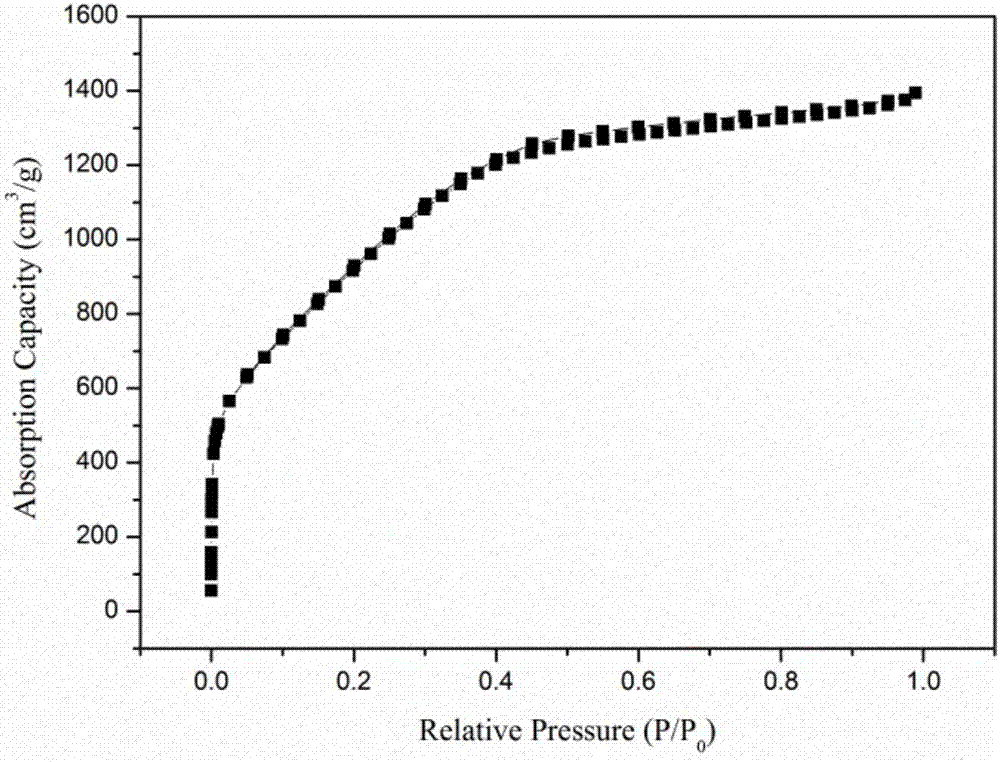
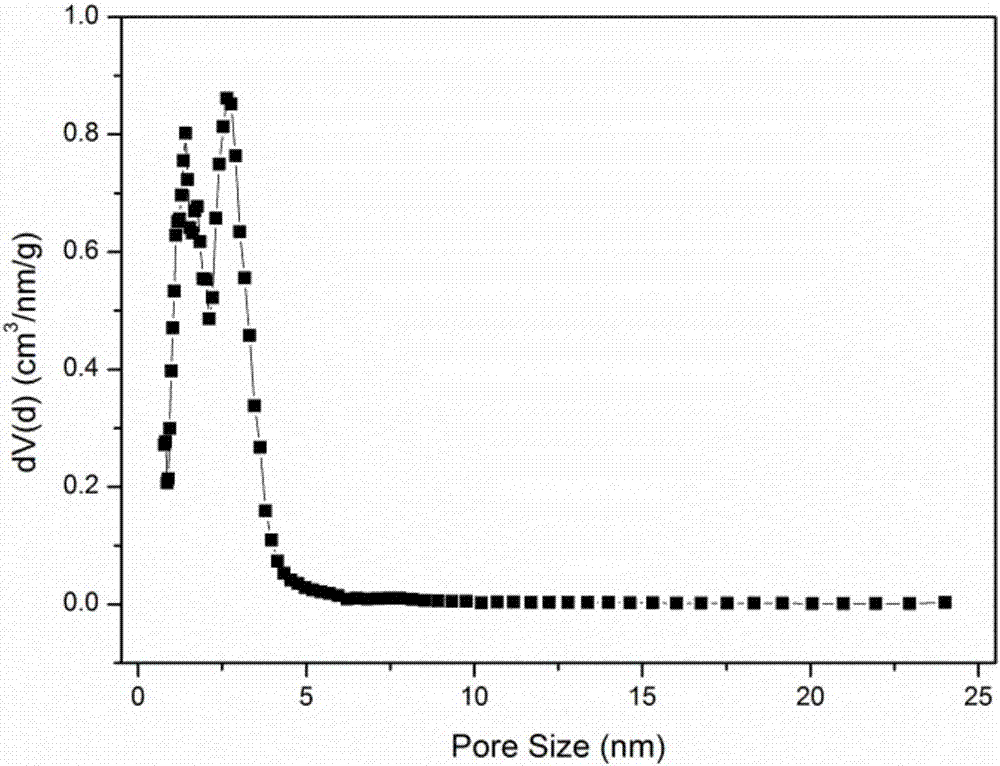
Image
Examples
Embodiment 1
[0036] Weigh 1.9g Ni(NO 3 ) 2 ·6H 2 O was fully dissolved in 120 mL of deionized water to prepare a nickel salt solution. Soak 8 g of clean rice stalks in the nickel nitrate aqueous solution for 12 hours, and transfer to a blast oven at 60° C. for drying. The dried sample was placed in a tube furnace, and the temperature was raised to 400° C. for 1 h at a rate of 5° C. / min in a nitrogen atmosphere to obtain a carbonized precursor. Weigh 3g of the carbonized precursor and soak it in 36mL of 6M KOH solution for 2h, and then transfer it to a 60°C blast oven for drying. The dried sample was placed in a tube furnace, heated to 700°C at a rate of 10°C / min under a nitrogen atmosphere and kept for 1 hour, and then cooled with the furnace. Soak the obtained material in pure water for 12 hours, filter and wash until neutral; then soak the material in 1M HNO 3 After 12 hours in the solution, filter, and finally wash with pure water to neutrality, dry the obtained material in a blast...
Embodiment 2
[0041] Weigh 0.3g Ni(NO 3 ) 2 ·6H 2O was fully dissolved in 120 mL deionized water to prepare a nickel salt solution. Soak 8g of clean rice stalks in nickel nitrate solution for 12h, and transfer them to a blast oven at 60°C for drying. Place the dried sample in a tube furnace, heat it up to 500°C at a rate of 5°C / min in a nitrogen atmosphere and keep it warm for 1 hour to obtain a carbonized precursor material; weigh 3g of the carbonized precursor and soak it into 36mL of 6M KOH solution 2h, and then transferred to 60 ℃ blast oven for drying. The dried samples were placed in a tube furnace and heated to 800 °C for 1 h at a rate of 10 °C / min under a nitrogen atmosphere, and then cooled with the furnace. Soak the obtained material in pure water for 12h, filter and wash until neutral, and then soak the material in 1M HNO 3 In the solution for 12 hours, filtered, and finally washed with pure water to neutrality, the obtained material was dried in a blast oven at 100°C to obt...
Embodiment 3
[0044] Weigh 1.6g C 4 h 6 o 4 Ni·4H 2 O (nickel acetate) was fully dissolved in 120mL deionized water to prepare a nickel salt solution. Soak 8g of clean wheat straw in nickel acetate solution for 12h, and transfer to a blast oven at 60°C for drying. Place the dried sample in a tube furnace, and heat it up to 400°C at a rate of 10°C / min in a nitrogen atmosphere for 1 hour to obtain a carbonized precursor material; weigh 3g of the carbonized precursor and soak it into 54mL of 6M KOH solution 2h, and then transferred to 60 ℃ blast oven for drying. The dried samples were placed in a tube furnace and heated to 900 °C for 1 h at a rate of 10 °C / min under a nitrogen atmosphere, and then cooled with the furnace. Soak the obtained material in pure water for 12 hours, filter and wash until neutral, then soak the material in 1M HCl solution for 12 hours, filter, and finally wash with pure water until neutral, then place the obtained material in a blast oven at 100°C After drying, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com