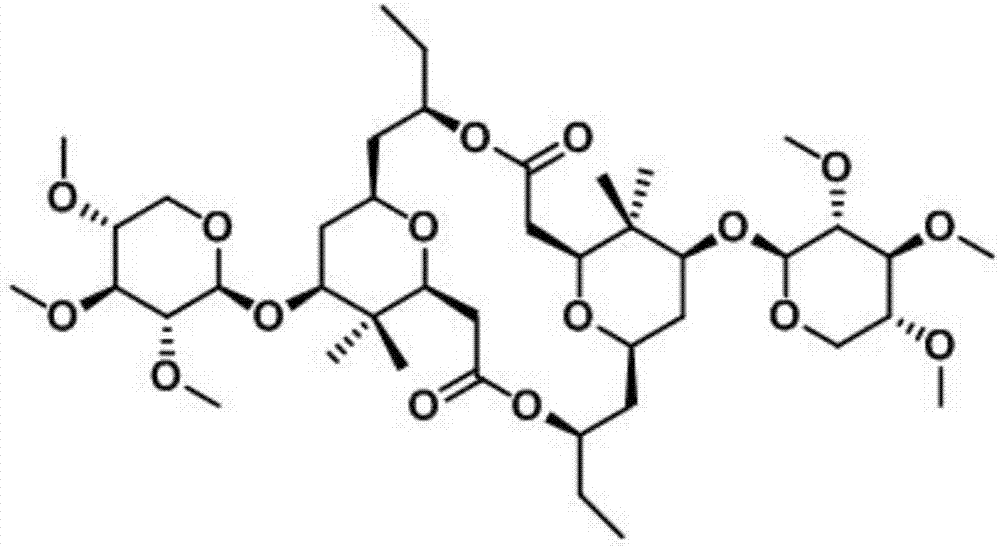
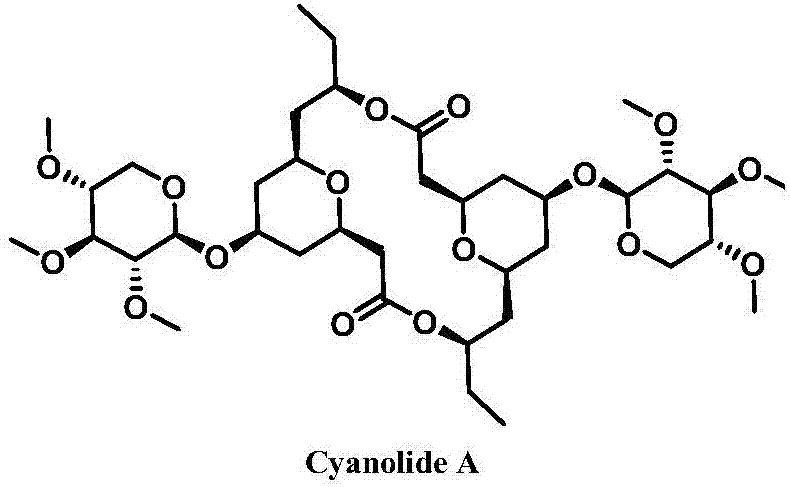
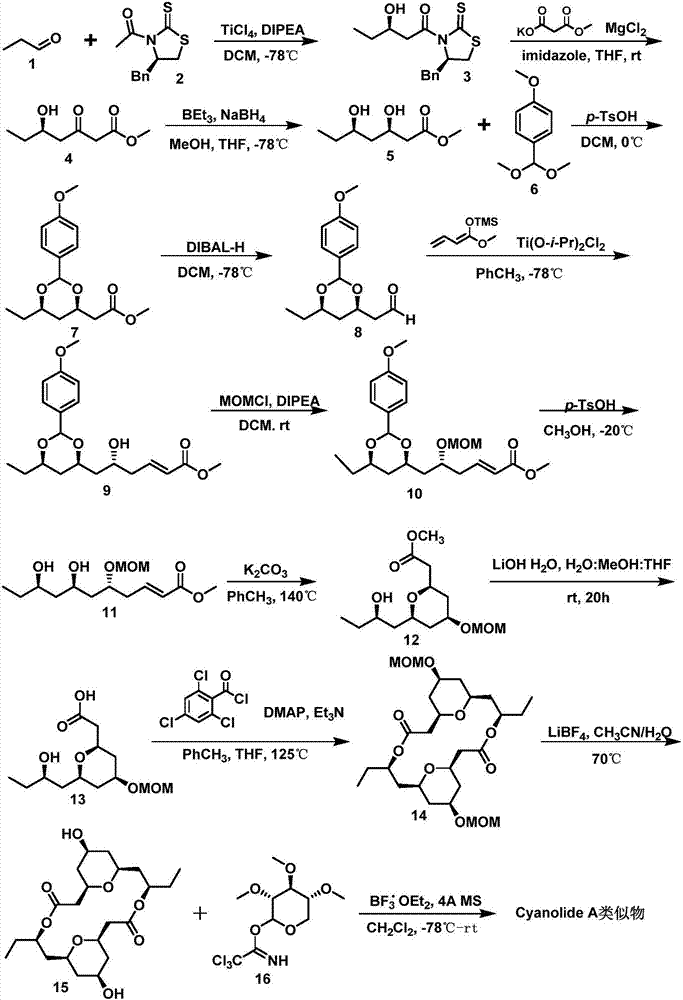
Novel method for synthesizing natural product Cyanolide A analogue
A technology of natural products and analogues, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high synthesis cost and low yield, achieve simple operation, reduce production cost, and increase yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the synthesis of formula 3 compound
[0021] Under the protection of nitrogen, the chiral auxiliary formula 2 (1.19g, 4.75mmol) was dissolved in 31ml of dry dichloromethane, and then the reaction system was placed in an ice-water mixture and cooled to 0°C, and 1.04ml of tetrachloride was slowly added dropwise. Titanium chloride. Stir at 0°C for 5 minutes, dissolve 1.65ml of DIPEA (diisopropylethylamine) in 3ml of dry dichloromethane and add to the reaction system. Then the reaction system was moved to -78°C. After reacting for 30 minutes, a solution of propionaldehyde formula 1 (1ml, 14.25mmol) in dry dichloromethane (9ml) was added dropwise, and the reaction system continued to react at -78°C for 3h. . After TLC (thin layer chromatography) detection reaction is complete, with saturated NH 4 After the Cl solution was quenched, the reaction system was moved to room temperature. After the solution temperature rose to room temperature, it was extracted wit...
Embodiment 2
[0022] Embodiment 2: the synthesis of formula 4 compound
[0023] Under the protection of nitrogen, the raw material formula 3 (2.8297g, 9.14mmol) was dissolved in 36.56ml of dry tetrahydrofuran, the reaction system was placed in an ice-water mixture and cooled to 0°C, and then monomethyl malonate potassium salt ( 2.85g, 18.28mmol), imidazole (1.24g, 18.28mmol) and anhydrous magnesium chloride (1.74g, 18.28mmol), the reaction system was reacted at room temperature for 44h, and saturated NH 4 The Cl solution was quenched, extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain the crude product, which was separated and purified by silica gel column chromatography to obtain 1.2106 g of the compound of formula 4 in a yellow viscous shape. rate 76%; 1 H NMR (400MHz, CDCl 3 ):δ3.99(s,1H),3.72(s,3H),3.48(s,2H),2.78-2.75(m,1H),2.74-2.59(m,2H),1.57-1.40(m,2H ),0.93(t,J=7.4Hz,3H); ...
Embodiment 3
[0024] Embodiment 3: the synthesis of formula 5 compounds
[0025] Under the protection of nitrogen, the raw material formula 4 (414.4mg, 2.38mmol) was dissolved in 14ml of dry tetrahydrofuran, the reaction system was placed in an ice-water mixture and cooled to 0°C, and then 7.14ml of triethylboron was added dropwise. After ethyl boron was added dropwise, 7.2ml of refined methanol was added dropwise, and the reaction was continued for 1h. Then the reaction system was moved to -78°C, and 269.99 mg of NaBH was added 4 , stirred at this temperature for 1 hour, and TLC detected that the reaction was completely completed. with saturated NH 4 The Cl solution was quenched, extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain a crude product. Then add 15ml of ethyl acetate to dissolve, add 20mL of 3% hydrogen peroxide solution dropwise, after TLC detects that the reaction is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com