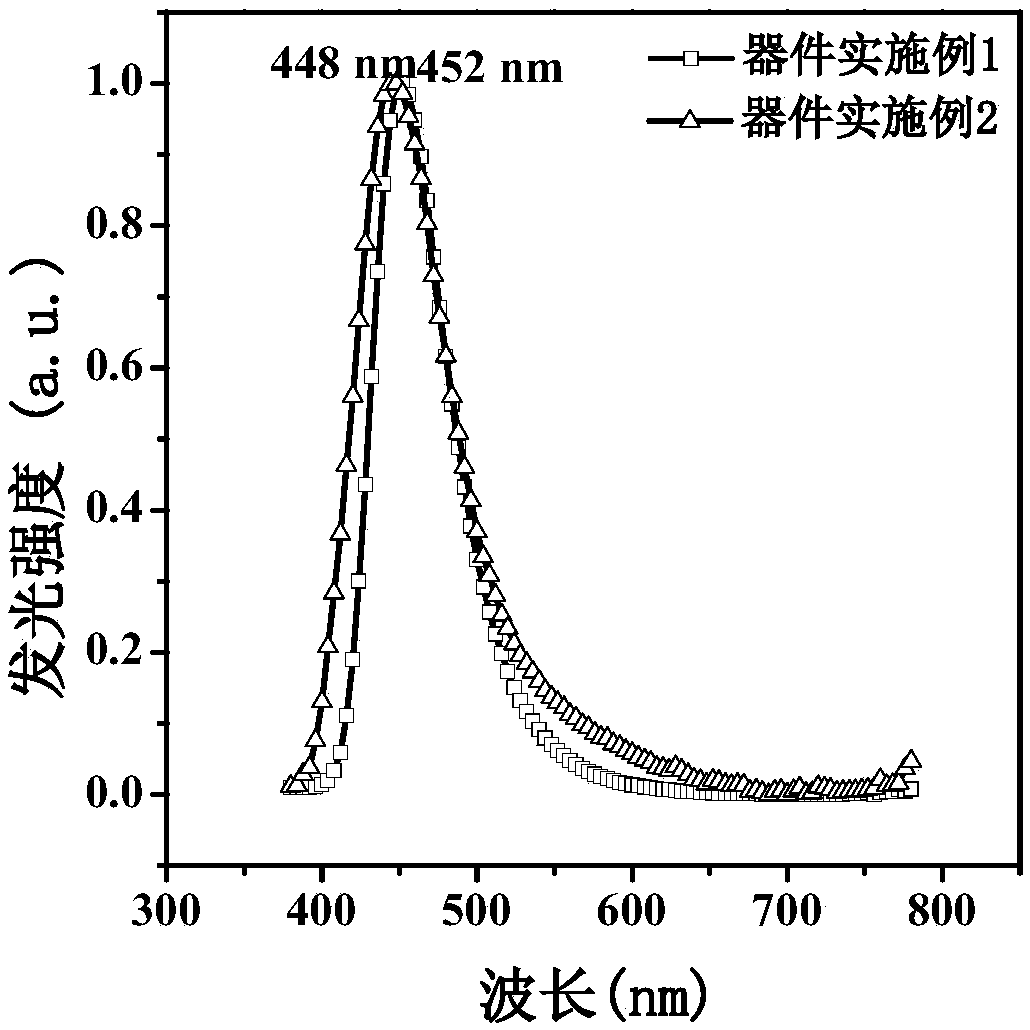
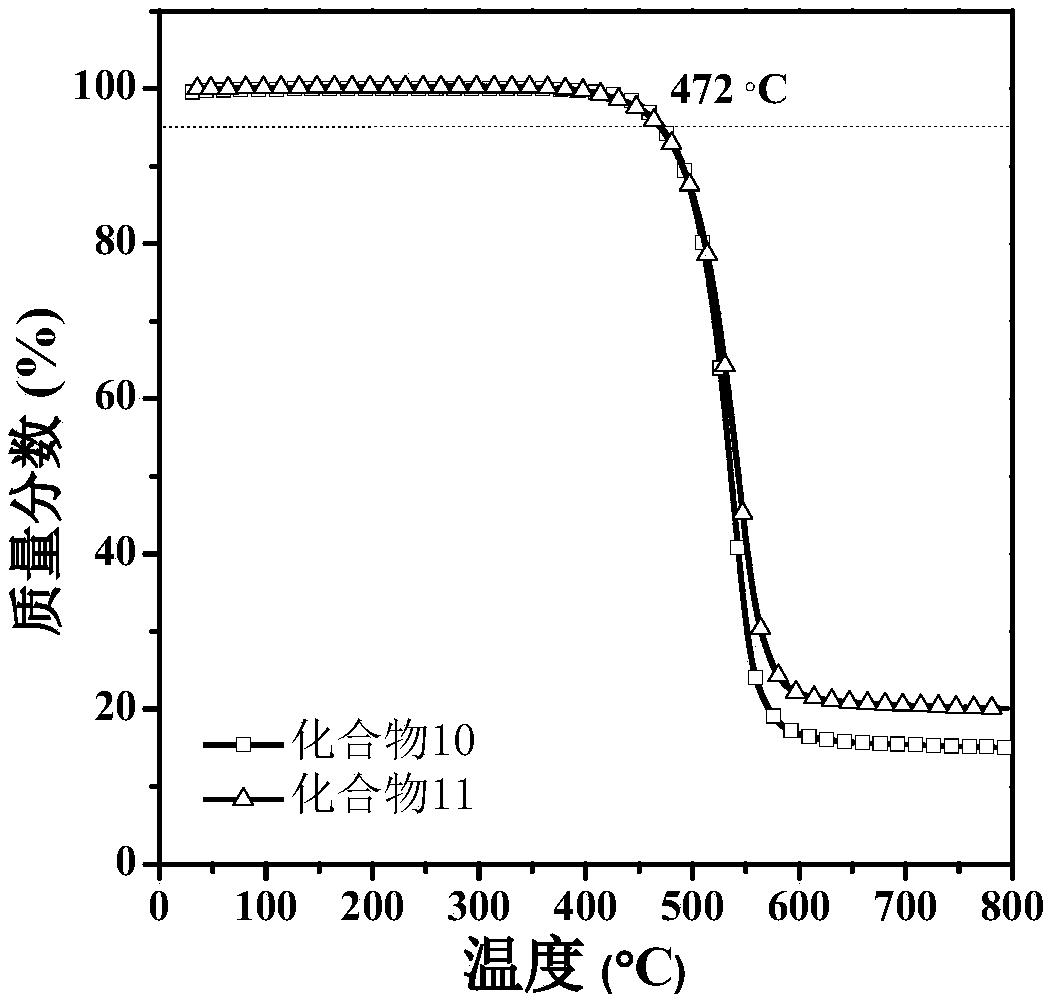
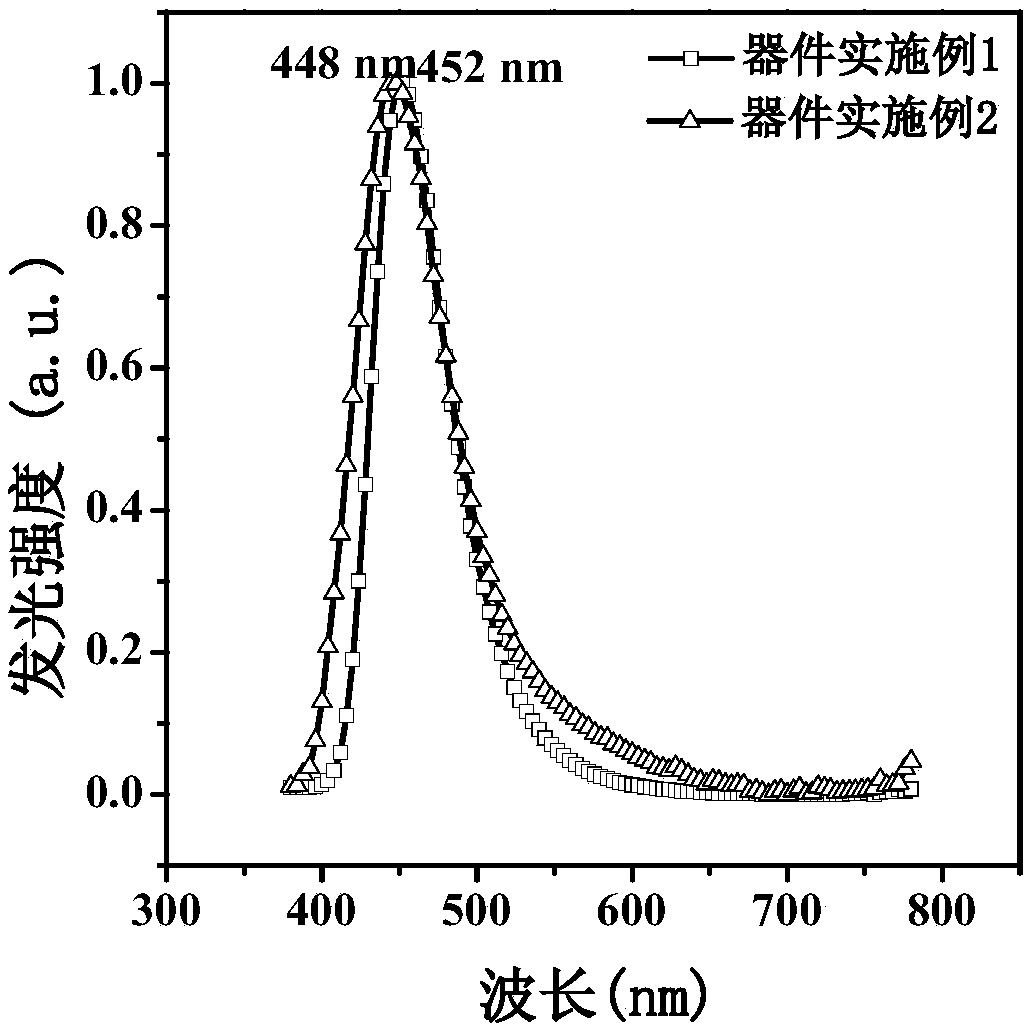
Pyrene and imidazole derivative and preparation method and application thereof
A technology of pyrenimidazole and its derivatives, which is applied in the field of pyrenimidazole derivatives and its preparation, and can solve problems such as unreachable luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The synthesis of embodiment 1 compound 1:
[0083] (1) Add elemental pyrene (4.02g) and sodium periodate (20g) into a 500mL round bottom flask, add 100mL distilled water and 100mL acetonitrile solution, add catalyst RuCl 3 The reaction was carried out at 40° C. for 24 hours. After separation and purification, orange powdery 4,5-pyrene diquinone was obtained.
[0084] (2) Take 4,5-pyrenediquinone (2.32g, 10mmol), aniline (4.65g, 50mmol), 4-bromobenzaldehyde (5.46g, 30mmol) and ammonium acetate (3.85g, 50mmol) in a 250mL round bottom In the flask, react at 120° C. for 3 hours under nitrogen protection. Then distilled water was added to the reaction system, and the precipitated crude product was obtained by filtration, which was separated by column chromatography to obtain 9-(4-bromo)-9-hydro-pyreneimidazole.
[0085] (3) Take 9-(4-bromo)-9-hydrogen-pyrene imidazole (4.73g), biboronic acid pinacol ester (3.81g) and newly prepared potassium carbonate solution (16mL, 2mol...
Embodiment 2
[0087] The synthesis of embodiment 2 compound 2
[0088] Step (1) was prepared according to the synthesis method of compound 1 step (1) in Example 1. Step (2) is prepared by using 4,5-pyrenediquinone, benzaldehyde and 4-bromoaniline as raw materials according to the synthesis method of compound 1 step (2) in Example 1. Step (3) is prepared by using 10-(4-bromo)-9-hydrogen-pyrenimidazole and biboronic acid pinacol ester as raw materials according to the synthesis method of compound 1 step (3) in Example 1. Step (4) is prepared by using 10-(4-pinacol borate)-9-hydro-pyrenezimidazole and bromobenzene as raw materials according to the synthesis method of compound 1 step (4) in Example 1. Using MALDI-TOF to identify the compound, the molecular formula is C 35 h 22 N 2 , detected value [M+]=470.19, theoretical value 470.56.
Embodiment 3
[0089] The synthesis of embodiment 3 compound 3
[0090] Step (1) was prepared according to the synthesis method of compound 1 step (1) in Example 1. Step (2) is prepared by using 4,5-pyrenediquinone, 4-bromobenzaldehyde and 4-bromoaniline as raw materials according to the synthesis method of compound 1 step (2) in Example 1. Step (3) is prepared by using 9,10-(4-bromo)-9-hydro-pyrene imidazole and biboronic acid pinacol ester as raw materials according to the synthetic method of compound 1 step (3) in Example 1. Step (4) is prepared by using 9,10-(4-pinacol borate)-9-hydro-pyrenimidazole and bromobenzene as raw materials according to the synthesis method of compound 1 step (4) in Example 1. Using MALDI-TOF to identify the compound, the molecular formula is C 41 h 26 N 2 , detected value [M+]=546.83, theoretical value 546.66.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com