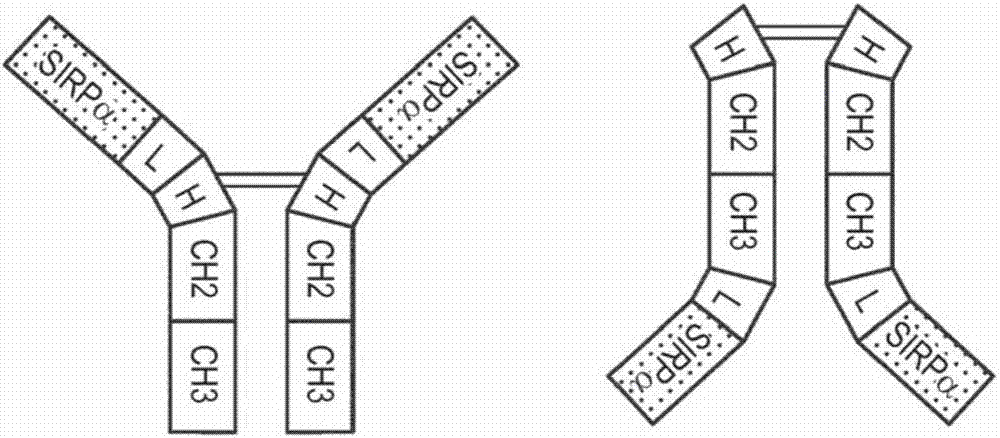
Sirp-alpha immunoglobulin fusion proteins
A technology of immunoglobulin and fusion protein, applied in the direction of immunoglobulin, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., which can solve the reverse immune control mechanism And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Example 1: Effect of anti-CD47 B6H12 on cynomolgus monkey erythrocytes
[0186] The in vivo effect of an anti-CD47 B6H12 monoclonal antibody (chimeric B6H12-human IgG4 (Lindberg et al., JBC 269: 1567, 1994)) on red blood cells (RBC) was evaluated in cynomolgus monkeys. A group of 3 monkeys received a single intravenous dose of 12 mg / kg of B6H12 on day 0. Blood samples were taken for RBC counts and hematocrit (HCT) determinations at -10 (10 days prior to injection to obtain baseline levels), 0, 1, 3, 5 and 7 days after the single intravenous administration. Figure 2A -B shows that on day 5 ( Figure 2A ), red blood cells from 5.8×10 9 Drastically reduced to 3.7×10 9 RBC / mL, a 40% decrease, and a corresponding decrease in hematocrit levels ( Figure 2B ). Therefore, treatment with anti-CD47 antibodies can lead to severe anemia.
Embodiment 2
[0187] Example 2: Anti-CD20-huIgG1-SIRPα immunoglobulin fusion protein
[0188] 2 (A) Construction and expression of anti-CD20-huIgG1-SIRPα
[0189] An exemplary anti-CD20-huIgG1-SIRPα generation was based on an anti-CD202B8 (rituximab) monoclonal antibody (Reff et al., Blood 83:435, 1994) and the SIRPα protein (Jiang et al., JBC 274:559, 1999). The DNA and protein sequences of the Fab light chain of 2B8 are provided in SEQ ID NO: 1 and SEQ ID NO: 2, respectively. The DNA and protein sequences of the Fab heavy chain of 2B8 are provided in SEQ ID NO: 3 and SEQ ID NO: 4, respectively. The DNA and protein sequences of SIRPα allele V1 are provided in SEQ ID NO: 5 and SEQ ID NO: 6, respectively. The DNA and protein sequences of the IgV domain of SIRPα allele V2 are provided in SEQ ID NO: 7 and SEQ ID NO: 8, respectively. By via (G4S) 4 A linker joined the C-terminus of the anti-CD20 heavy chain polypeptide to the IgV domain of SIRPαV2 to generate anti-CD20-huIgG1-SIRPαV2.
[...
Embodiment 3
[0201] Example 3: Anti-CD20 / anti-CD47 bispecific antibody
[0202]3(A) Description of anti-CD20 / anti-CD47
[0203] Generation of an exemplary tetravalent bispecific antibody (TetBiAb) against CD20 and CD47 was based on the anti-CD202B8 (rituximab) monoclonal antibody (Reff et al., Blood 83:435, 1994) and the anti-CD47 B6H12 monoclonal antibody ( Lindberg et al., JBC 269:1567, 1994). In anti-CD20 / anti-CD47 TetBiAbs against CD20 and CD47, the C-terminus of the anti-CD20 heavy chain polypeptide was linked to the N-terminus of the anti-CD47 Fab light chain via a G4S linker ( Figure 1D +E). International Patent Application Publication No. WO2014 / 144357 describes the construction, expression, and binding properties of anti-CD20 / anti-CD47.
[0204] 3(B) In vivo biological activity of anti-CD20 / anti-CD47
[0205] The following in vivo experiments used anti-CD20 / anti-CD47 as a surrogate for anti-CD20-huIgG1-SIRPα. In a diffuse lymphoma model, SCID mice were injected intravenously...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com