Triple transgenic pigs suitable for xenograft
A technology for transgenic pigs, genes, applied in the field of xenotransplantation and genetic modification, which can solve problems such as expensive maintenance drugs, increased infection risk, weight gain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
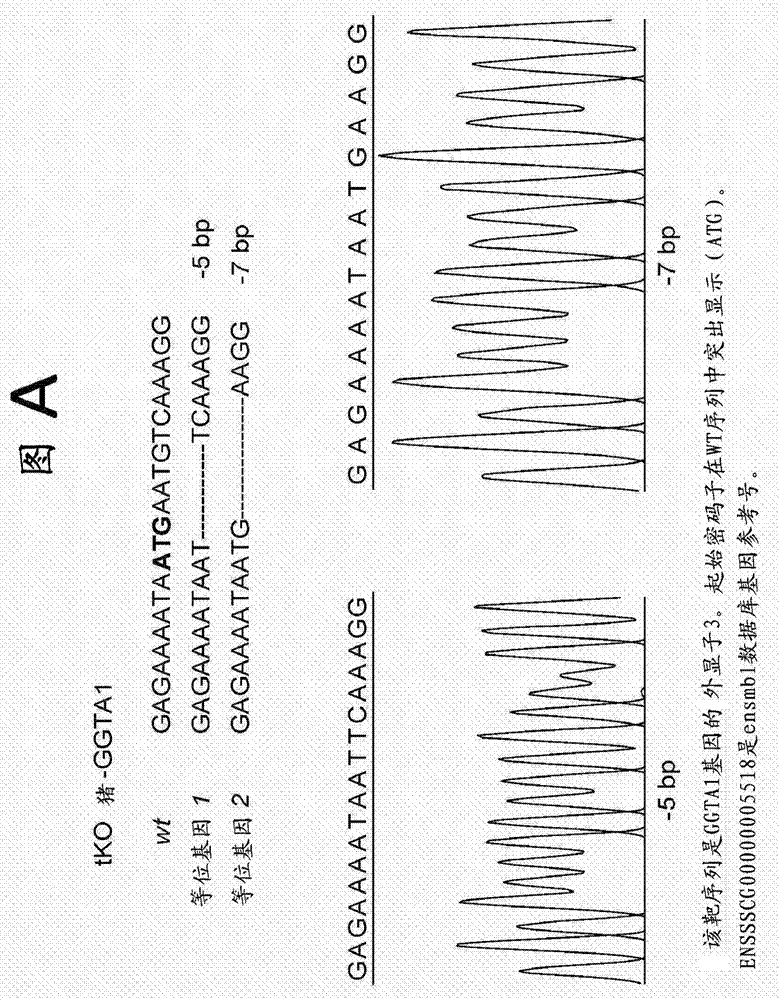
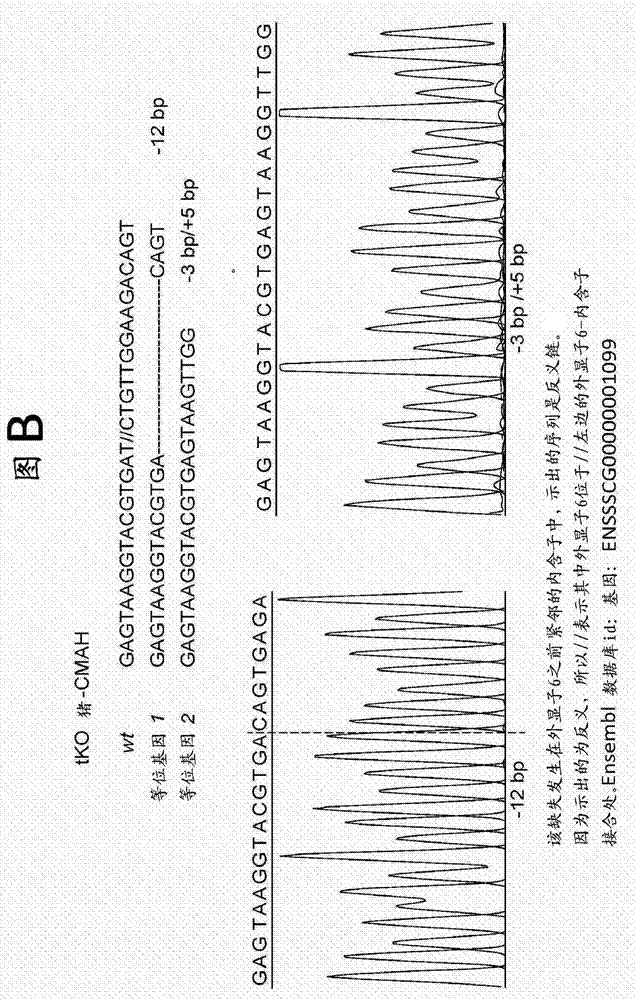
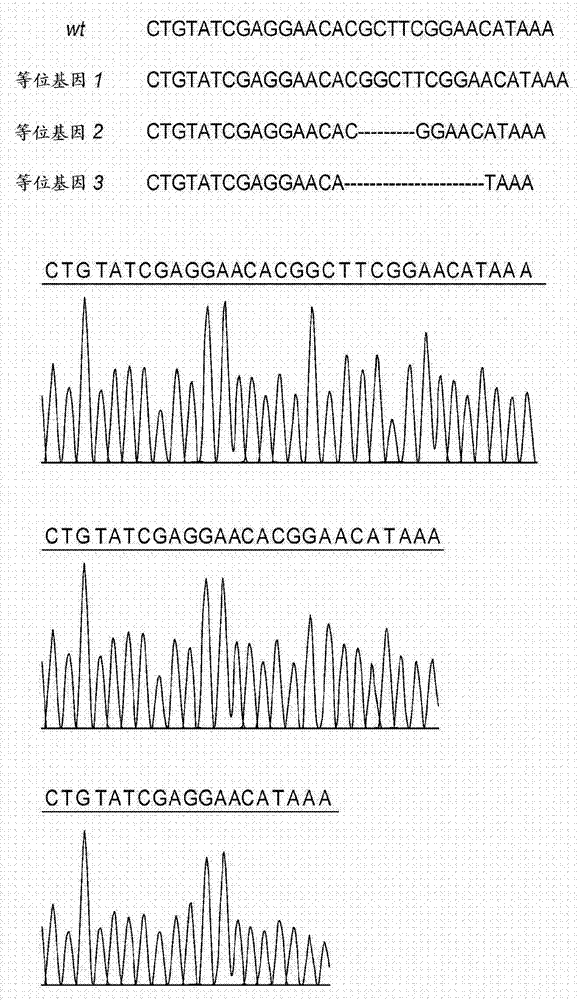
[0106] Example 1. DNA sequencing analysis of targeted CMAH, GGTA1 and β4GalNT2 regions
[0107] Genomic DNA from cloned pigs was extracted using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO). PCR amplification of CMAH, GGTA1 and β4GalNT2 Crispr / Cas9 target regions was performed. Primers were used to sequence the targeted CMAH, GGTA1 and β4GalNT2 regions.
[0108] Using Pwo Master (Roche, Indianapolis IN), Pwo SuperYield DNA polymerase, dNTPack (Roche Applied Science, Indianapolis, IN) was used. The PCR conditions for GGTA1 were as follows: 94°C, 2 minutes; 94°C, 15 seconds, 54°C, 30 seconds, and 72°C, 45 seconds, for 15 cycles; 94°C, 15 seconds, 54°C, 30 seconds, 72°C , 45 seconds, plus 5 seconds per cycle, for 25 cycles; and a final extension step of 72° C. for 5 minutes. For CMAH, 94°C for 2 minutes; 94°C for 15 seconds, 56°C for 30 seconds and 72°C for 45 seconds for 15 cycles; 94°C for 15 seconds, 56°C for 30 seconds, 72°C for 45 secon...
Embodiment 2
[0110] Example 2. Generation of knockout pigs (triple transgenic pigs)
[0111] Oligonucleotide annealing was performed using Addgene plasmid 42230 [http: / / www.addgene.org / 42230 and 20] and cloned into the PX330 plasmid to drive gRNA expression. The oligonucleotide pair targeting the gene is GGTA1 (NCB1 accession number: XM_005660398.1), 5'CACCGAGAGAAAATAATGAATGTCAA-3' forward) (SEQ ID NO: 8), 5'AAATTGACATTCATTATTTTCTC-3' (reverse) (SEQ ID NO: 9); CMAH (NCBI accession number: NM_001113015.1) 5'-CACCGAG AAGGTACGTGATCTGT-3' (forward) (SEQ ID NO: 10), 5'-AAACACAGATCACGTACCTTACTC-3' (reverse) (SEQ ID NO: 11; β4GalNT2 (NCBI accession number NM_001244330.1) 5'-CACCGTGTATCGAGGAACACGCTT-3' (forward) (SEQ ID NO: 12), 5'-AAACAAGCGTGTTCCTCGATACAC-3' (reverse) (SEQ ID NO: 13 ).
[0112] Hepatic-derived cells were co-transfected with all three gRNA / Cas9 plasmids. After 48 hours, the treated cells were passed through an IB4 lectin column to isolate α-Gal null cells. Two million α-Gal ...
Embodiment 3
[0115] Example 3. IB4 counter-selection for triple knockout
[0116] Liver-derived cells (LDCs) were transfected with three sets of targeting constructs (αGal, β4GalNT2 and CMAH). Cells were selected with IB4, a substance that binds αGal. Cellular DNA from a large population of cells surviving IB4 counter-selection was obtained and evaluated for target gene sequences. A large population of cells surviving the IB4 counter-selection was used directly for SCNT to make pregnant pigs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com