Phenylurea-substituted n-thioacylhomoserine lactone compounds, preparation method and application thereof
A technology of thioacyl homoserine and ester compounds, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: prepare derivative (2-a) shown by general formula 2
[0033] Dissolve homoserine lactone hydrochloride (150 mg, 1.09 mmol) in 8 mL of acetone, then add trisodium phosphate dodecahydrate (620 mg, 1.63 mmol) and CS 2 (160μL), stirred at room temperature for 2h. Then, add 2-chloro-N-((2-methoxyphenyl)carbamoyl)acetamide (291mg, 1.20mmol), react at room temperature for 6h, evaporate the system to dryness, extract with ethyl acetate and water, and wash the organic layer with water After spin-drying, the crude product was separated by silica gel column chromatography (eluent: ethyl acetate / petroleum ether=1 / 1) to obtain 271 mg of compound (2-a) as a light yellow solid, with a yield of 65%.
Embodiment 2
[0034] Embodiment 2: Preparation of derivatives (2-b, 2-c) shown by general formula 4
[0035] Using 2-chloro-N-(naphthyl-1-carbamoyl)acetamide instead of 2-chloro-N-((2-methoxyphenyl)carbamoyl)acetamide, prepared by the method described in Example 1 Compound 2-b.
[0036] Substitute 2-chloro-N-((3-tolyl)carbamoyl)acetamide for 2-chloro-N-((2-methoxyphenyl)carbamoyl)acetamide by the method described in Example 1 Compound 2-c was prepared.
[0037] The chemical structure of the partial preferred compound that the present invention synthesizes, nuclear magnetic data is as follows table 1:
[0038]
[0039]
[0040]
Embodiment 3
[0041] Embodiment 3: In vitro anti-quorum sensing screening test of compounds of the present invention
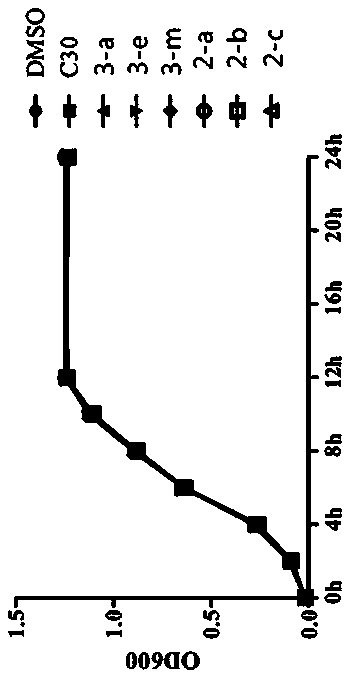
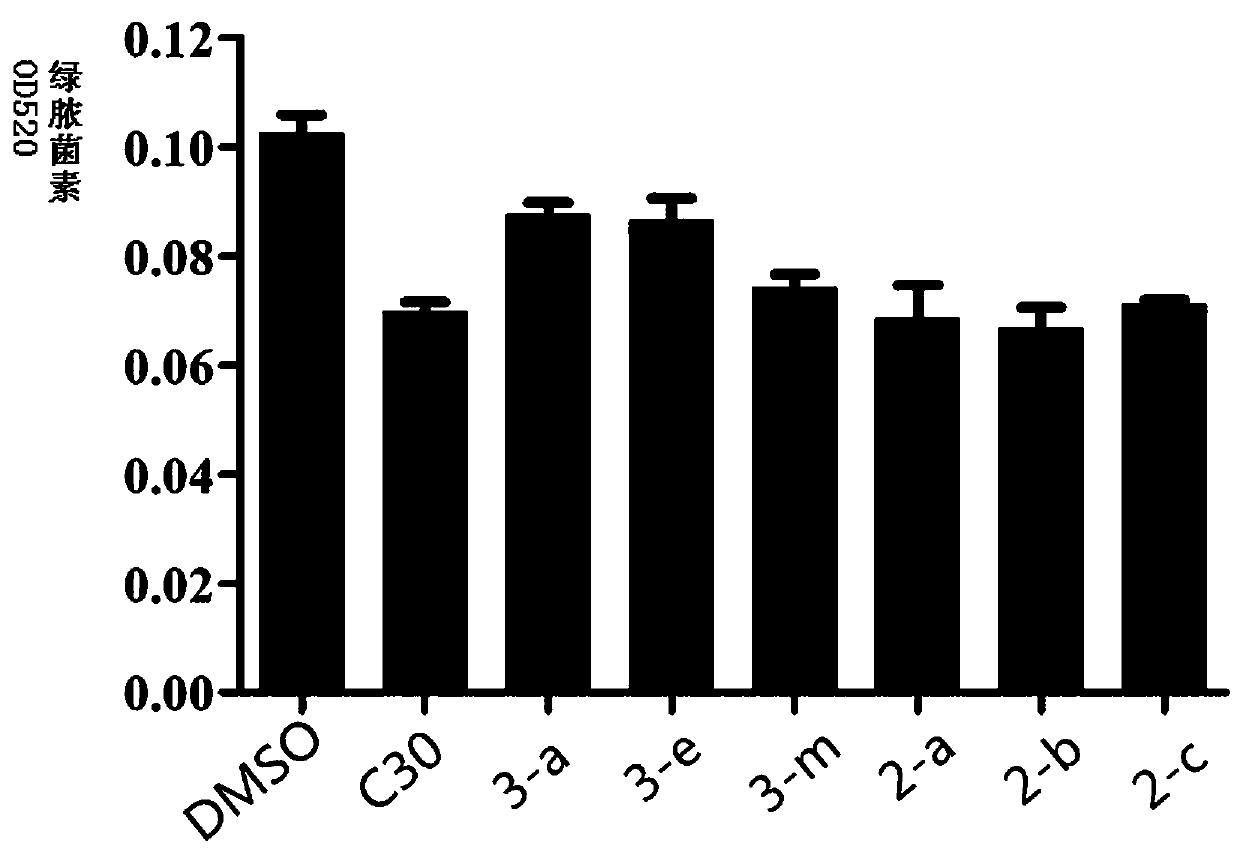
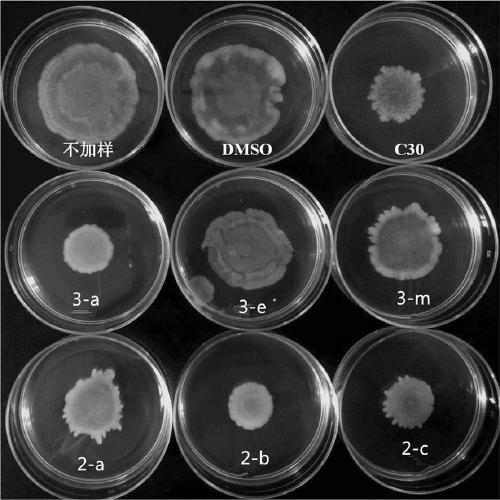
[0042] The effect of the test compound on the growth of Pseudomonas aeruginosa PAO1, at the sub-inhibitory concentration, the test compound on the effect of Pseudomonas aeruginosa pyocyanin and Swarming movement (see Figure 1- image 3 ). To detect the effect of compounds on the mRNA expression levels of genes related to the quorum sensing system of Pseudomonas aeruginosa PAO1, that is, to detect the mRNA expression levels of QS-related genes in Pseudomonas aeruginosa PAOI by real-time fluorescent quantitative PCR. These genes are mainly related to bacterial synthesis of signal molecules, biofilm formation, including lasI, lasR, pqsA, pqsR, rhlI, rhlR (see Figure 4-Figure 9 ).
[0043] The experimental results show that the compound of the present invention has more obvious quorum sensing inhibitory activity than the compound shown in the patent CN 2016109869810. Under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com