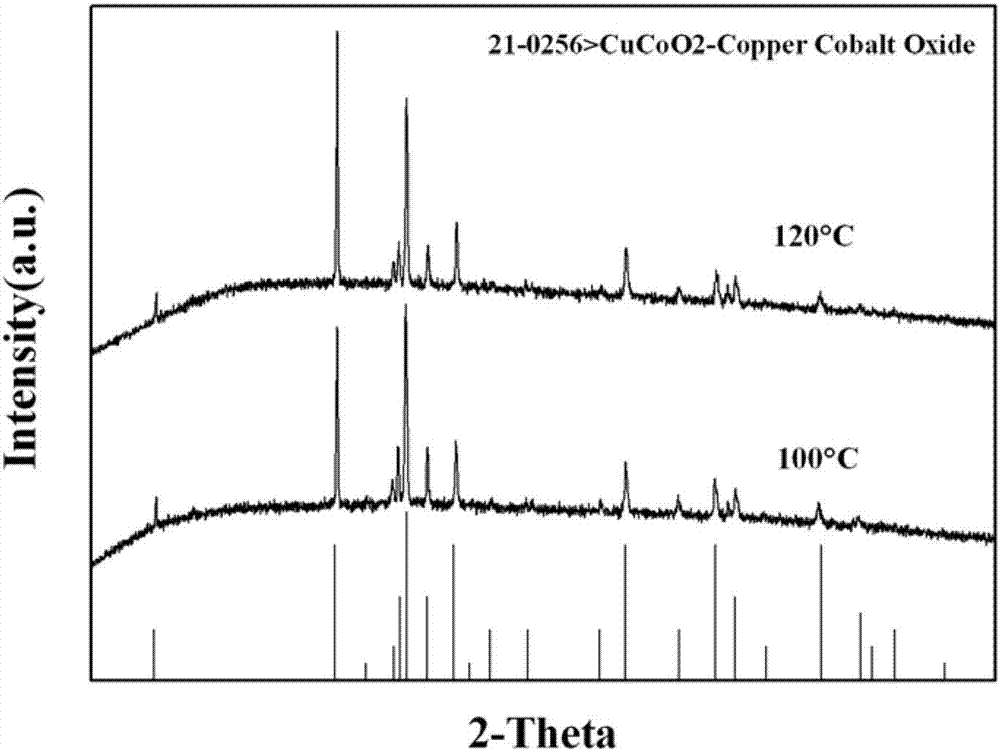
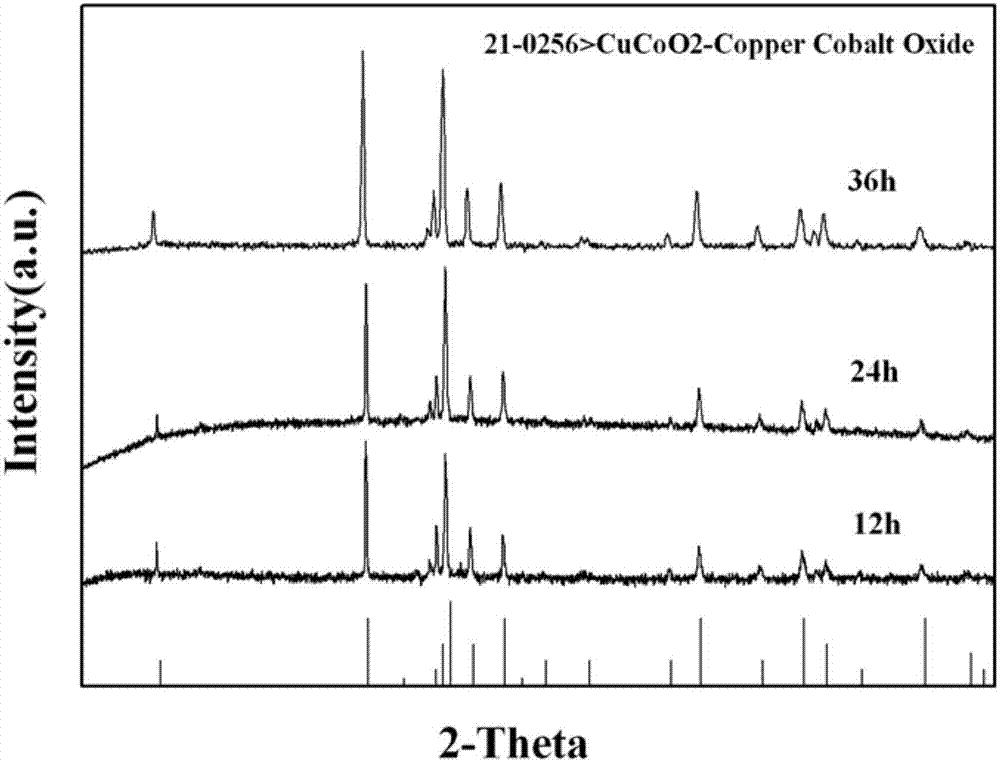
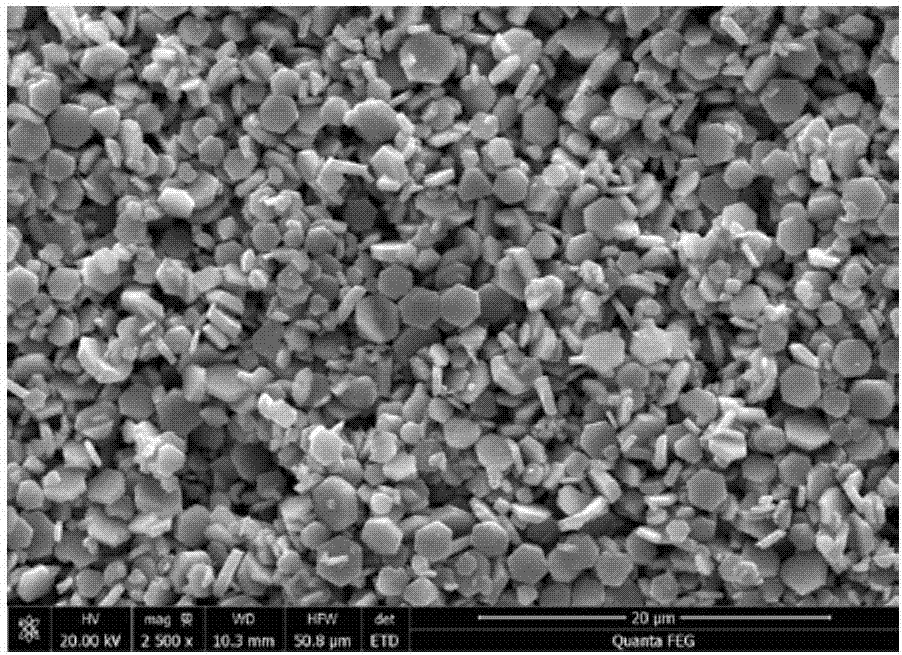
A delafossite-structured CuCoO2 crystal material and a low-temperature preparation method thereof
A technology of crystalline material and delafossite, applied in chemical instruments and methods, inorganic chemistry, cobalt compounds, etc., can solve the problems that have not been found so far, and achieve the effects of less environmental pollution, simple preparation process and wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] At room temperature, according to Co 2+ : Cu 2+ Weigh Co(NO 3 ) 2 ·6H 2 O and Cu(NO 3 ) 2 ·3H 2 After adding O into pure water, Cu 2+ and Co 2+ The concentrations are 4.33-4.92Wt%, 5.17-5.87Wt%, respectively, stirred by a magnetic stirrer for 30-60 minutes; 2+ or contain Co 2+ 4 times the molar amount of NaOH of the compound, and continue to stir for 30-60 minutes until completely dissolved to form a hydrothermal reaction precursor and obtain a reaction precursor. The above-mentioned reaction precursor is transferred to a hydrothermal reactor (generally polytetrafluoroethylene), and the filling rate of the reaction solution (ie, the reaction precursor) is controlled to be about 70%. After sealing the kettle body, place it in a program temperature-controlled box for hydrothermal reaction, set the reaction temperature to 100°C, and the reaction time to 24-36 hours.
[0037] After the reaction, the kettle body was naturally cooled to room temperature, and the ke...
Embodiment 2
[0039] At room temperature according to Co 2+ : Cu 2+ Weigh Co(NO 3 ) 2 ·6H 2 O and Cu(NO 3 ) 2 ·3H 2 After adding O into pure water, Cu 2+ and Co 2+ The concentrations are 4.33-4.92Wt%, 5.17-5.87Wt%, respectively, stirred by a magnetic stirrer for 30-60 minutes, and after being completely dissolved, then add Cu-containing mineralizer 2+ or contain Co 2+ 5 times the molar amount of NaOH of the compound, and continue to stir for 30-60 minutes until completely dissolved to form a hydrothermal reaction precursor and obtain a reaction precursor. Transfer the above reaction precursor to a hydrothermal reactor (generally polytetrafluoroethylene), and control the filling rate of the reaction solution to about 70%. After sealing the kettle body, place it in a program temperature-controlled box for hydrothermal reaction, set the reaction temperature to 100°C, and the reaction time to be 12 to 36 hours.
[0040] After the reaction, the kettle body was naturally cooled to room...
Embodiment 3
[0042] At room temperature according to Co 2+ : Cu 2+ Weigh Co(NO 3 ) 2 ·6H 2 O and Cu(NO 3 ) 2 ·3H 2 After adding O into pure water, Cu 2+ and Co 2+ The concentrations are 4.33-4.92Wt%, 5.17-5.87Wt%, respectively, stirred by a magnetic stirrer for 30-60 minutes, and after being completely dissolved, then add Cu-containing mineralizer 2+ or contain Co 2+ 6 times the molar amount of the compound (NaOH), and continue to stir for 30 to 60 minutes until completely dissolved to form a hydrothermal reaction precursor and obtain a reaction precursor.
[0043] Transfer the above reaction precursor to a hydrothermal reactor (generally polytetrafluoroethylene), and control the filling rate of the reaction solution to about 70%. After sealing the kettle body, place it in a program temperature-controlled box for hydrothermal reaction, set the reaction temperature to 100-120°C, and the reaction time to 24 hours.
[0044] After the reaction, the kettle body was naturally cooled t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com