A composite protein of chimeric antigen receptor fused with inducible apoptosis enzyme
A technology of chimeric antigen receptors and complex proteins, which is applied in the fields of genetic engineering and oncology, can solve the problems of patients' body injury, cell off-target effects, etc., and achieve the effect of avoiding the storm of inflammatory factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Construction of a plasmid with a specific receptor protein that binds to the Her2 receptor, simultaneously strengthens the activation of immune T lymphocytes, and can regulate apoptosis
[0046] 1.1 Primer design: After finding the Her2 single-chain fragment antibody from the gene library, the activation fragment (CD8α chain, CD137 transmembrane segment, CD3ζ intracellular segment), and the sequence of the induction fragment (the conserved segment of Fkbp12 and caspase-9), use Primer software designed relevant primers and connecting primers respectively. The entire field sequence of the above-mentioned fragments can be searched and obtained from Gene Bank.
[0047] 1.1.1. Her2-ScFv primers:
[0048] Forward: NcoI is an endonuclease
[0049] 5'-GGGCCATGGCCCAGGTGCAGCTGTTGCAGTCTGGGGCAGAG-3' (SEQ ID NO: 3)
[0050] Reverse: NotI is an endonuclease
[0051] 5'-TTGCGGCCGCTCCGGAATTCACCTAGGACGGTCAGCTTGGTCCC-3' (SEQ ID NO: 4)
[0052] 1.1.2. Primers for activating...
Embodiment 2
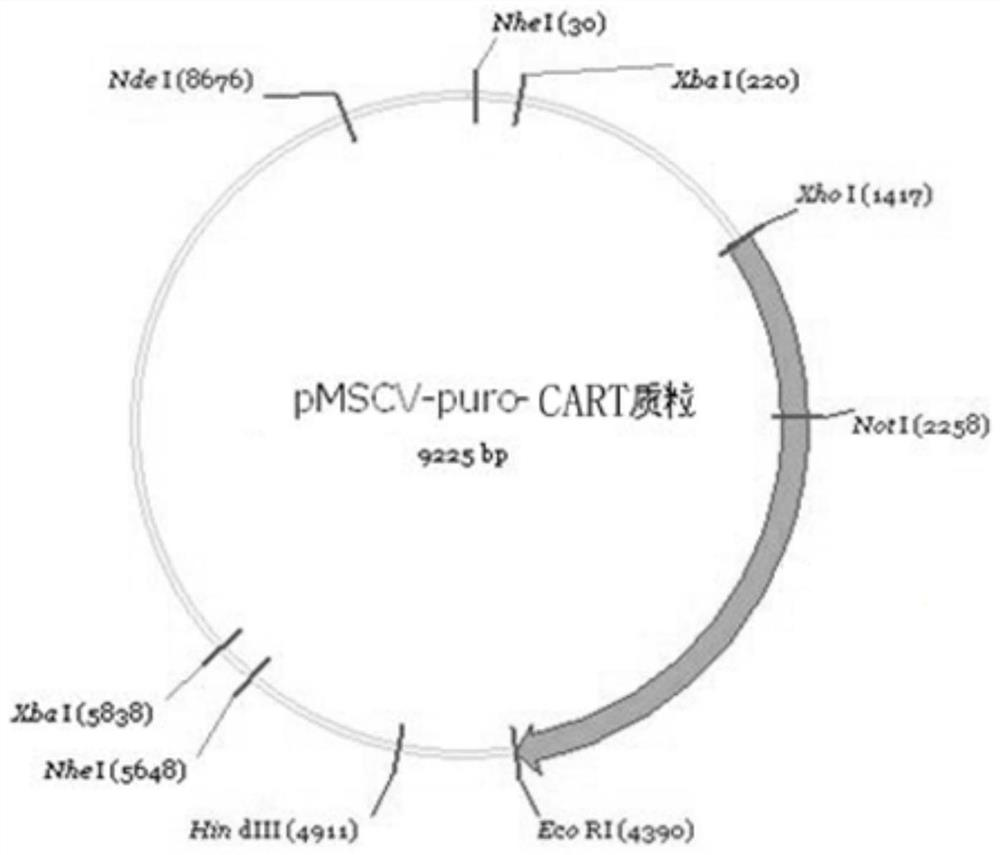
[0114] Example 2 Small amount extraction of pMSCV puro-Her2-scfv-CD8α-CD137-CD3ζ-iCasp9 plasmid
[0115] 2.1 Bacteria collection: Collect bacteria by centrifugation at 12000g×1min. (Generally, 3-4ml bacterial liquid is used for extracting one portion);
[0116] 2.2 Discard the supernatant;
[0117] 2.3 Add 250u l ice-bath Buffer S1 (containing RNase A), vortex and oscillate to fully suspend the bacteria;
[0118] 2.4 Add 250ul Buffer S2, mix gently 6 times (this process does not exceed 5min); add 350ul Buffer S3, mix gently 6 times;
[0119] 2.5 Centrifuge at 12000g×10min;
[0120] 2.6 Take the supernatant, pass it through the DNA preparation tube, 12000g×1min, and discard the filtrate;
[0121] 2.7 Add 700ul Buffer W1 to the DNA preparation tube, 12000g×1min, discard the filtrate;
[0122] 2.8 Add 500ul BufferW2 to the DNA preparation tube, 12000g×1min, discard the filtrate;
[0123] 2.9 Centrifuge again at 12000g×1min;
[0124] 2.10 plus 40ulddH 2 Put O (or EB buffer...
Embodiment 3
[0126] Example 3 Identification of Chimeric Receptor Fusion-Induced Apoptosis Complex Protein by Enzyme Digestion Plasmid pMSCVpuro-Her2-scfv-CD8α-CD137-CD3ζ-iCasp9
[0127] The pMSCV puro-Her2-scfv-CD8α-CD137-CD3ζ-iCasp9 plasmid was analyzed with the sequence analysis software BioEdit, and the position of each restriction site was correctly located.
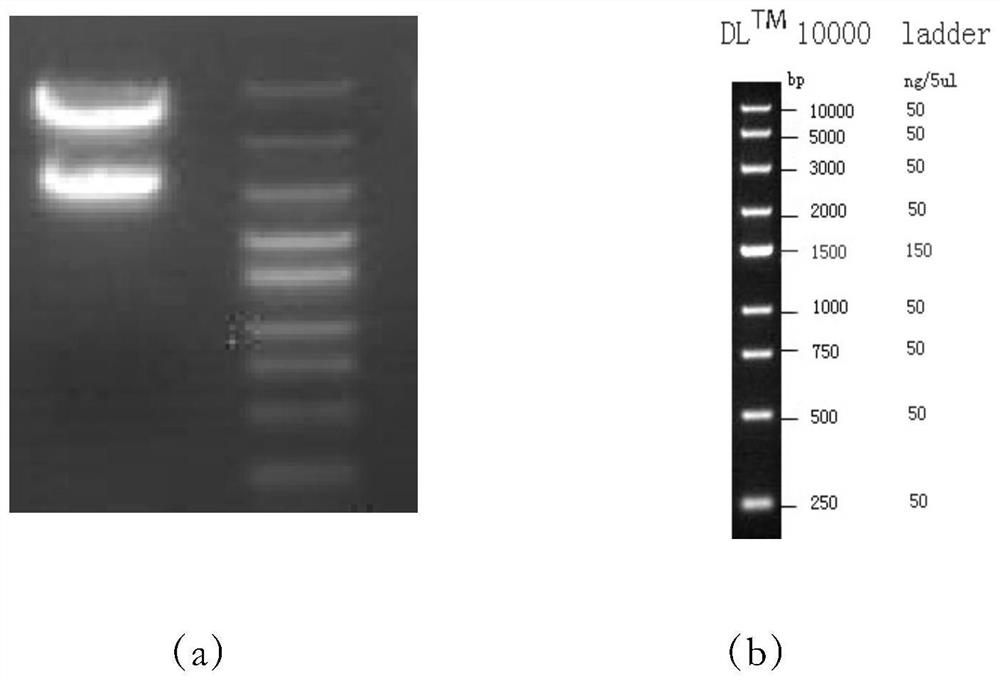
[0128] The restriction site of EcoRI (G^AATTC) is at 4537; the restriction site of XhoI (C^TCGAG) is at 1417; Dicer will cut from the 3 bases before and after the restriction site, therefore, the cut band size will be 6 bases more than the actual insertion sequence), and the position is accurate, such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com