An organic electroluminescent device containing 10,10-diarylanthrone compounds and its application
An electroluminescent device, diaryl anthrone technology, applied in the field of semiconductors, can solve difficult problems such as high exciton utilization rate and high fluorescence radiation efficiency, low S1 state radiation transition rate, efficiency roll-off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
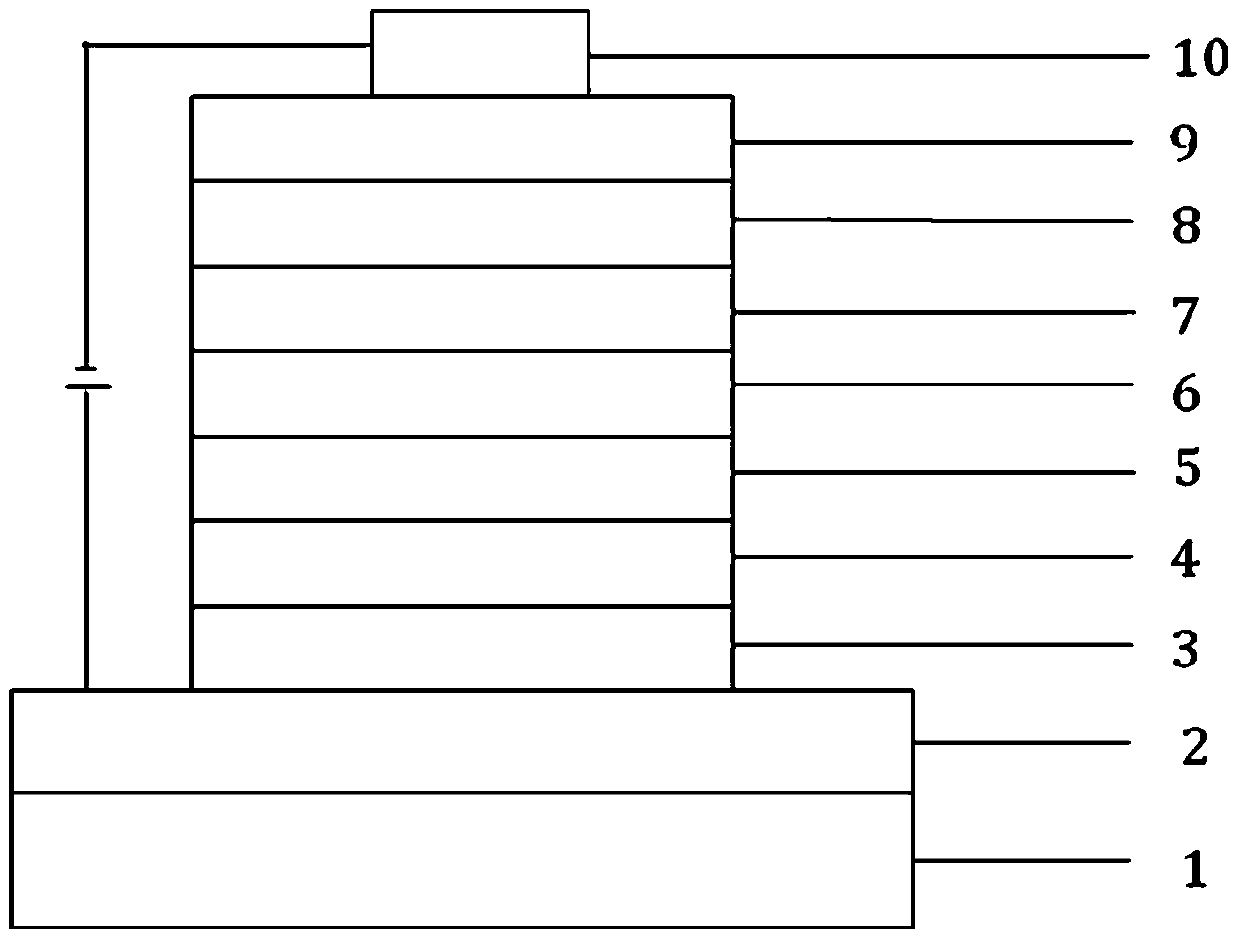
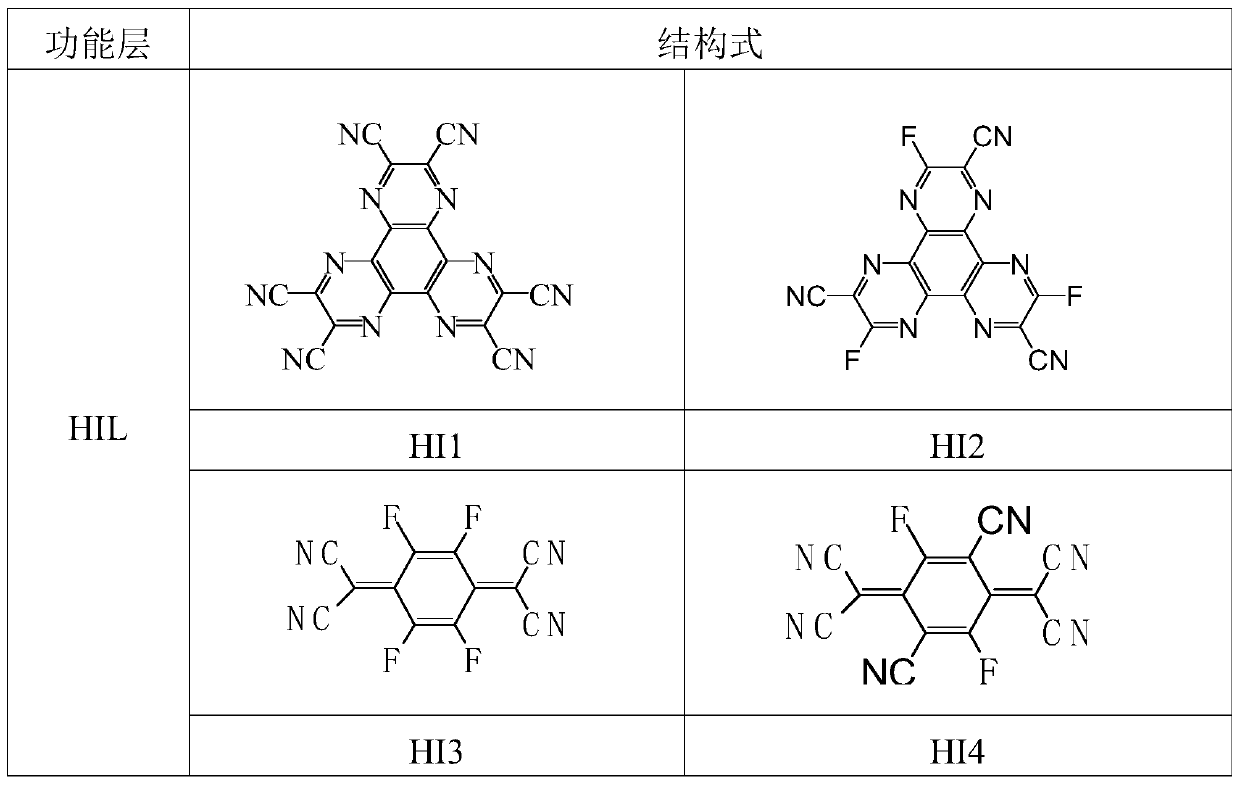
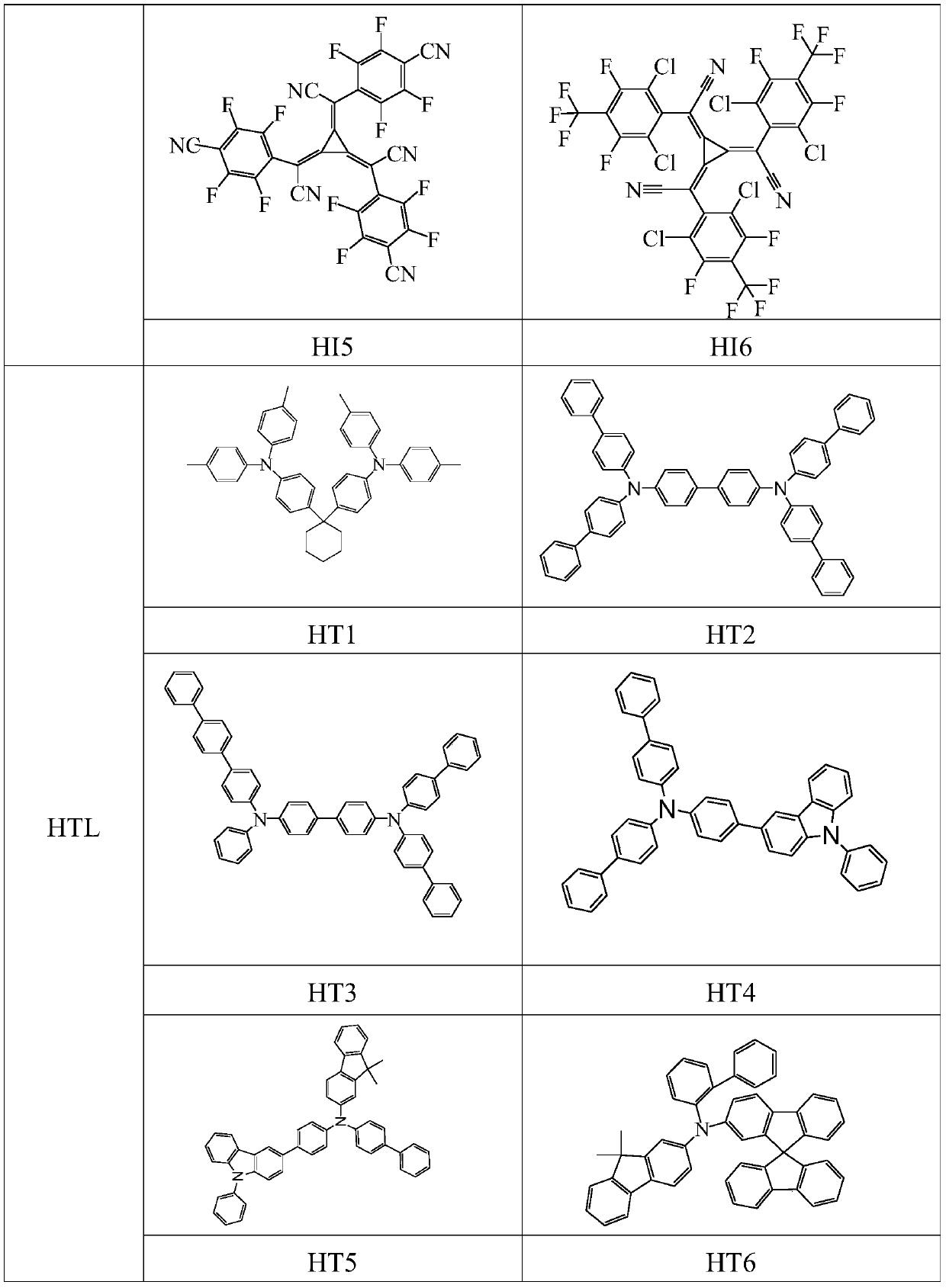
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 compound 1
[0068]
[0069] In a 1L three-necked flask equipped with a constant pressure dropping funnel, metal magnesium (5.1g, 0.21mol) was added, p-bromoiodobenzene (56.6g, 0.20mol) was dissolved in 300g tetrahydrofuran, and placed in a constant pressure drop In the funnel, use nitrogen protection, heat the three-necked flask until the temperature in the bottle reaches 65°C, add the tetrahydrofuran solution of p-bromoiodobenzene through the constant pressure dropping funnel, first add 50mL, after the reaction is triggered, slowly drop the remaining part, 1h After completion of the dropwise addition, react for 2 h at reflux temperature, and transfer to a constant pressure funnel after cooling down to room temperature for use;
[0070] Dissolve anthraquinone (41.6g, 0.20mol) in 200g tetrahydrofuran and add it to a 2L three-neck flask, then slowly add the above-mentioned solution for use dropwise, and react at reflux temperature for 3...
Embodiment 2
[0074] The preparation of embodiment 2 compound 2
[0075]
[0076] In a 250ml three-necked flask, under the protection of nitrogen, add 8.51g (0.02mol) of compound 1, 7.62g (0.03mol) of bis-boronic acid pinacol ester, 3.92g (0.04mol) of potassium acetate, 0.30g pd 2 (dba) 3 , 0.20g of tri-tert-butylphosphine, 100ml of toluene, heated to reflux for 20 hours, took a sample and spotted the plate, and the reaction was complete; naturally cooled, filtered, and the filtrate was rotary evaporated, and column chromatography gave compound 2 with a HPLC purity of 99.7% and a yield of 87.57%.
[0077] High-resolution mass spectrometry, ESI source, positive ion mode, molecular formula C32H29BO3, theoretical value 472.2210, test value 472.2215.
[0078] Elemental analysis (C32H29BO3): theoretical value C: 81.36, H: 6.19, O: 10.16, test value: C: 81.40, H: 6.15, O: 10.18.
Embodiment 3
[0079] The preparation of embodiment 3 compound 3
[0080]
[0081] In a 1L three-necked flask equipped with a constant-pressure dropping funnel, metal magnesium (5.1g, 0.21mol) was added, and s-tribromobenzene (63.0g, 0.2mol) was dissolved in 300g of tetrahydrofuran, and placed in a constant-pressure drop In the funnel, use nitrogen protection, heat the three-necked flask until the temperature in the bottle reaches 65°C, add the tetrahydrofuran solution of tribromobenzene through the constant pressure dropping funnel, first add 50mL, after the reaction is triggered, slowly add the remaining part, 1h After the dropwise addition, react at reflux temperature for 2 h, and transfer to a constant pressure funnel after cooling down to room temperature for use. Dissolve anthraquinone (41.6g, 0.2mol) in 200g tetrahydrofuran and add it to a 2L three-neck flask, then slowly add the above-mentioned solution for use dropwise, and react at reflux temperature for 3h. The solution was sl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com