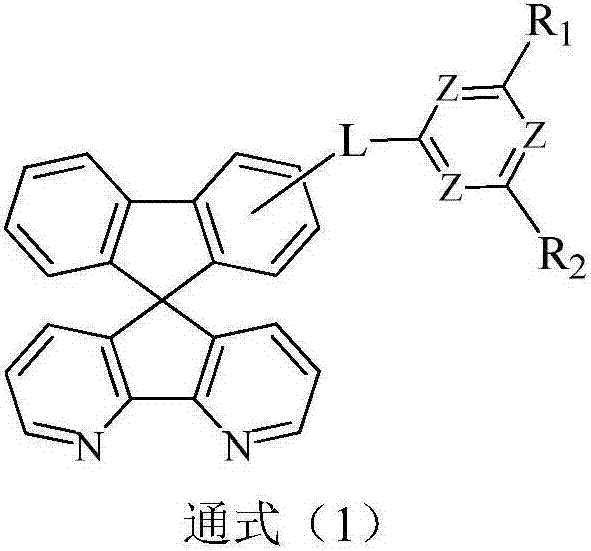
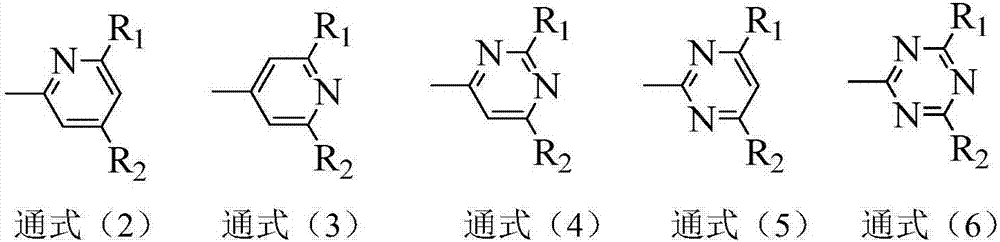
Compound containing aza-spirofluorene and nitrogen-containing hexa-heterocycle and application of compound in organic light emission diode device
An electroluminescent device, a technology of six-membered heterocycles, applied to organic electroluminescence devices, in the field of compounds containing azaspirofluorene and nitrogen-containing six-membered heterocycles, which can solve problems such as differences and achieve difficulty in aggregation , good industrialization prospects, good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
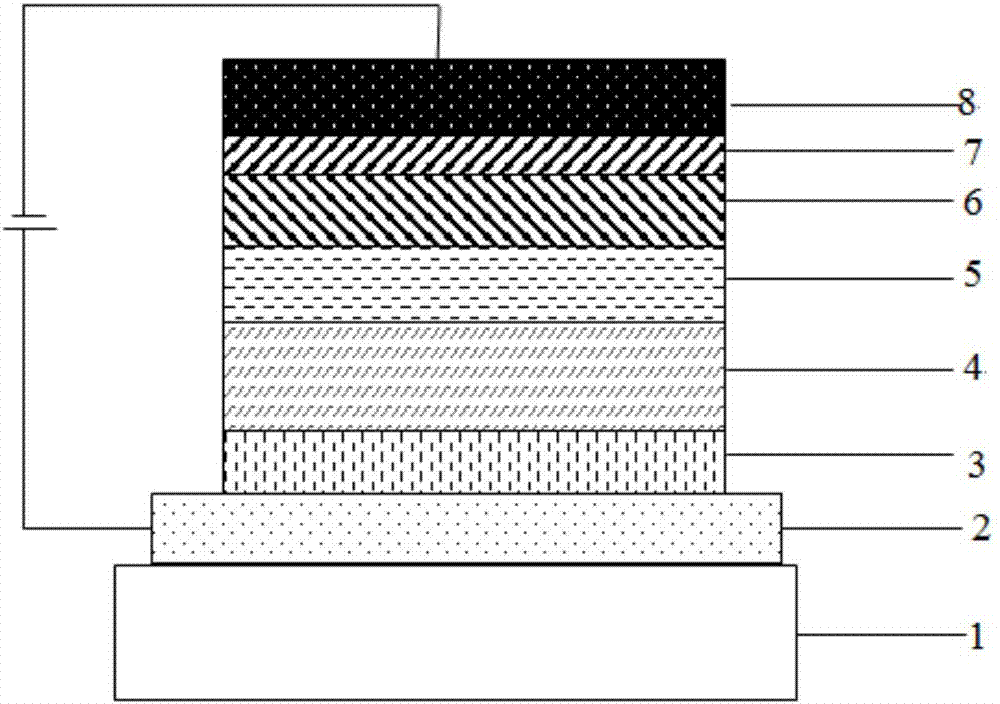
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of intermediate A:
[0040]
[0041](1) Under a nitrogen atmosphere, weigh raw material B and dissolve it in tetrahydrofuran, then add raw material C and tetrakis(triphenylphosphine)palladium, stir the mixture, then add potassium carbonate aqueous solution, and mix the above-mentioned reactants at the reaction temperature At 70-90°C, heat to reflux for 5-20 hours. After the reaction, cooling and adding water, the mixture was extracted with dichloromethane, the extract was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, the obtained residue was purified by silica gel column to obtain intermediate I;
[0042] The molar ratio of raw material B to raw material C is 1:1.0 to 1.5, the molar ratio of tetrakis(triphenylphosphine) palladium to raw material B is 0.001 to 0.02:1, and the molar ratio of potassium carbonate to raw material B is 1.0 to 2.0:1 , The ratio of THF dosage to raw material B is 1g:10~30m...
Embodiment 2
[0065] Embodiment 2: the synthesis of compound 2:
[0066]
[0067] In a 250mL three-necked flask, nitrogen was introduced, 0.01mol of raw material A1, 150ml of THF, 0.015mol of intermediate A1, 0.0001mol of tetrakis(triphenylphosphine) palladium were added, stirred, and then 0.02mol of K 2 CO 3 Aqueous solution (2M), heated to 80°C, refluxed for 15 hours, sampled and plated, the reaction was complete. Cool naturally, extract with 200ml of dichloromethane, separate layers, dry the extract with anhydrous sodium sulfate, filter, rotate the filtrate, and purify through a silica gel column to obtain the target compound with a HPLC purity of 99.1% and a yield of 77.3%. Elemental analysis structure (molecular formula C 44 h 27 N 5 ): theoretical value C, 84.46; H, 4.35; N, 11.19; test value: C, 84.47; H, 4.36; N, 11.17. ESI-MS(m / z)(M + ): The theoretical value is 625.23, and the measured value is 625.57.
Embodiment 3
[0068] Embodiment 3: the synthesis of compound 4:
[0069]
[0070] In a 250mL three-necked flask, feed nitrogen, add 0.01mol of raw material A1, 150ml of THF, 0.015mol of intermediate A2, 0.0001mol of tetrakis(triphenylphosphine)palladium, stir, and then add 0.02mol of K 2 CO 3 Aqueous solution (2M), heated to 80°C, refluxed for 15 hours, sampled and plated, the reaction was complete. Cool naturally, extract with 200ml of dichloromethane, separate layers, dry the extract with anhydrous sodium sulfate, filter, rotate the filtrate, and purify through a silica gel column to obtain the target compound with an HPLC purity of 99.3% and a yield of 71.9%. Elemental analysis structure (molecular formula C 50 h 31 N 5 ): theoretical value C, 85.57; H, 4.45; N, 9.98; test value: C, 85.57; H, 4.44; N, 9.99. ESI-MS(m / z)(M + ): The theoretical value is 701.26, and the measured value is 701.62.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com