Method for identifying aromatic isomers 2-hydroxybenzoic acid and 3-hydroxybenzoic acid
A technology of hydroxybenzoic acid and isomers, applied in the field of qualitative analytical chemistry, can solve the problems of differentiation and identification that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The present embodiment verifies the feasibility of the identification method of aromatic isomer 2-hydroxybenzoic acid and 3-hydroxybenzoic acid according to the following steps:
[0055] (1) Preparation solution
[0056] First prepare 0.026mol / L sulfuric acid with 98% concentrated sulfuric acid and distilled water as a stock solution, then use 0.026mol / L sulfuric acid solution as solvent to prepare respectively 0.13mol / L potassium iodate solution, 0.0183mol / L [NiL ](ClO 4 ) 2 Solution, 2.2mol / L malonic acid solution, 3.8mol / L hydrogen peroxide solution.
[0057] At the same time, double distilled water was used as solvent to prepare 0.12 mol / L solutions of 2-hydroxybenzoic acid and 3-hydroxybenzoic acid respectively.
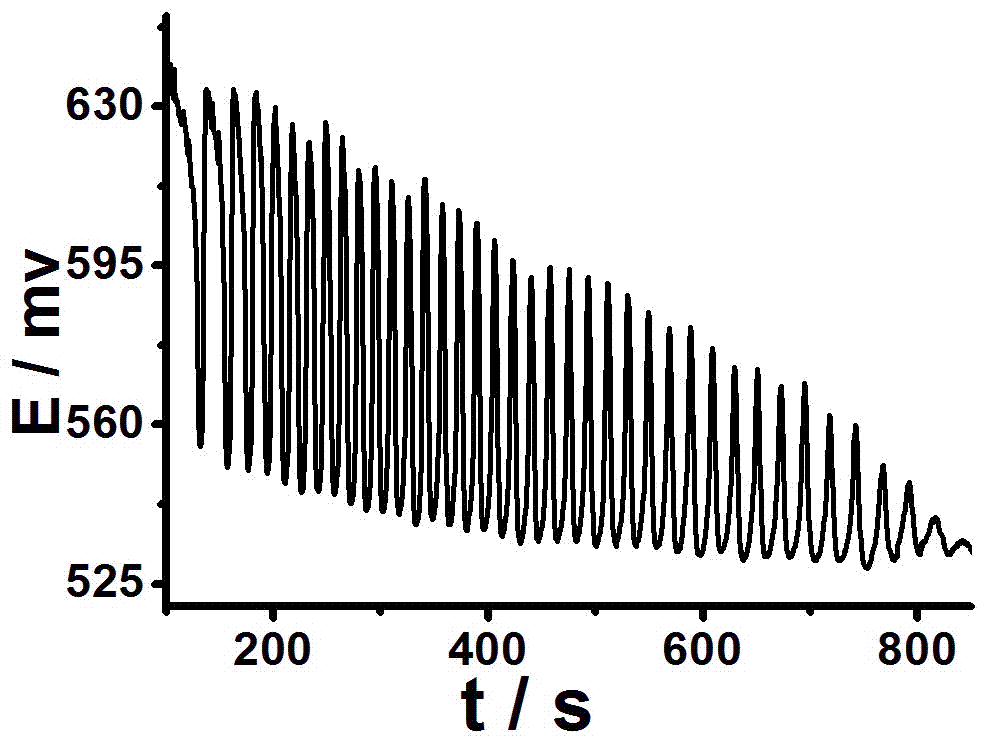
[0058] (2) Oscillation spectrum
[0059] Add magnetons to a 50mL small beaker, then add 15mL 0.026mol / L sulfuric acid solution, 6mL 0.13mol / L potassium iodate solution, 2mL 0.0183mol / L [NiL](ClO 4 ) 2 solution, 3mL of 2.2mol / L malonic acid, and fina...
Embodiment 2
[0069] The present embodiment verifies the feasibility of the identification method of aromatic isomer 2-hydroxybenzoic acid and 3-hydroxybenzoic acid according to the following steps:
[0070] (1) Preparation solution
[0071] First prepare 0.026mol / L sulfuric acid with 98% concentrated sulfuric acid and distilled water as a stock solution, then use 0.026mol / L sulfuric acid solution as solvent to prepare respectively 0.13mol / L potassium iodate solution, 0.0183mol / L [NiL ](ClO 4 ) 2 Solution, 2.2mol / L malonic acid solution, 3.8mol / L hydrogen peroxide solution.
[0072] At the same time, double distilled water was used as a solvent to prepare 0.14 mol / L solutions of 2-hydroxybenzoic acid and 3-hydroxybenzoic acid respectively.
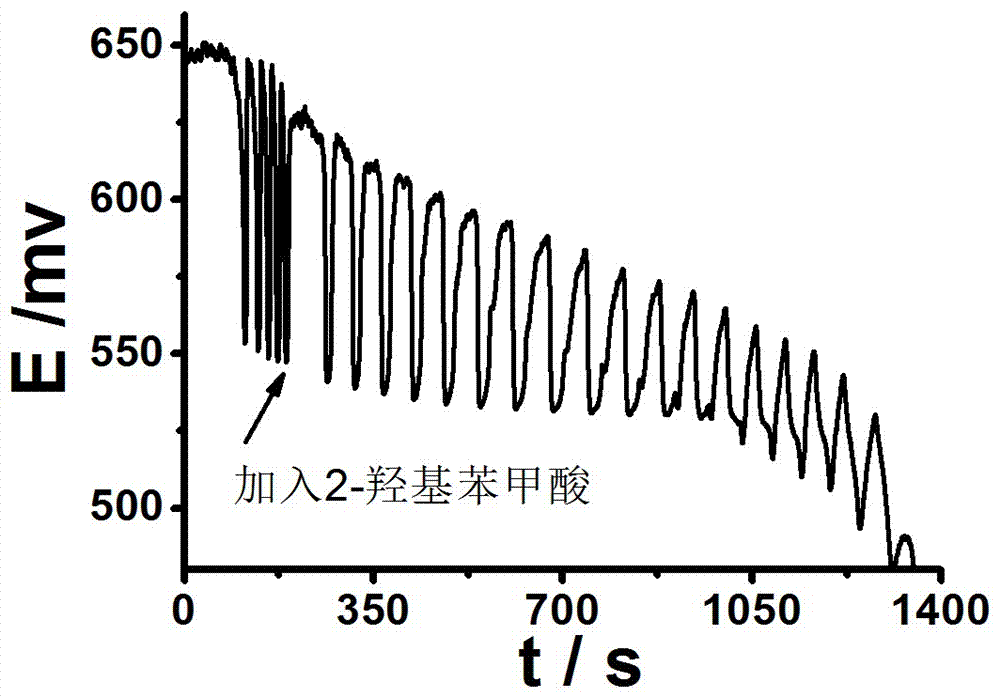
[0073] (2) Oscillation spectrum
[0074] Add a magnet to a 50mL small beaker, then add 5.5mL 0.026mol / L sulfuric acid solution, 10mL distilled water, 5.5mL 0.13mol / L potassium iodate solution, 2mL 0.0183mol / L [NiL](ClO 4 ) 2 solution, 3mL 2.2mol / L m...
Embodiment 3
[0084] The present embodiment verifies the feasibility of the identification method of aromatic isomer 2-hydroxybenzoic acid and 3-hydroxybenzoic acid according to the following steps:
[0085] (1) Preparation solution
[0086] First prepare 0.026mol / L sulfuric acid with 98% concentrated sulfuric acid and distilled water as a stock solution, then use 0.026mol / L sulfuric acid solution as solvent to prepare respectively 0.13mol / L potassium iodate solution, 0.0183mol / L [NiL ](ClO 4 ) 2 Solution, 2.2mol / L malonic acid solution, 3.8mol / L hydrogen peroxide solution.
[0087] At the same time, double distilled water was used as a solvent to prepare 0.2 mol / L solutions of 2-hydroxybenzoic acid and 3-hydroxybenzoic acid respectively.
[0088] (2) Oscillation spectrum
[0089] Add magnetons to a 50mL small beaker, then add 15mL 0.026mol / L sulfuric acid solution, 6mL 0.13mol / L potassium iodate solution, 1.5mL 0.0183mol / L [NiL](ClO 4 ) 2 solution, 3.5mL 2.2mol / L malonic acid was dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com