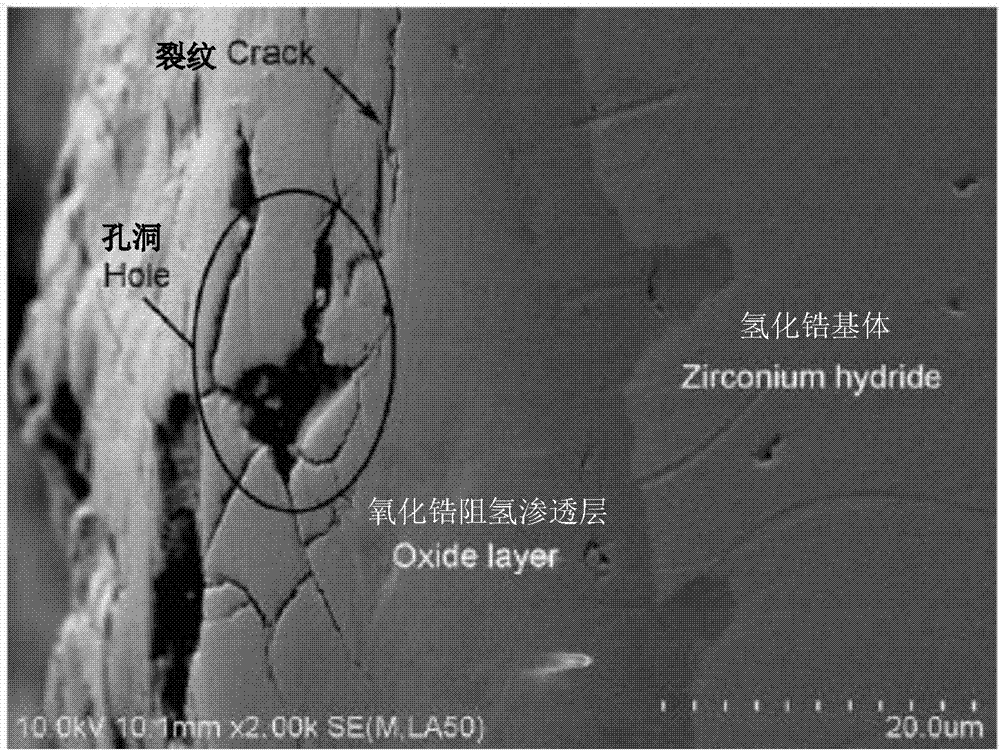
Composite hydrogen resisting permeating layer on surface of metal hydride and preparation method thereof
A technology of surface compounding and hydride, which is applied in the direction of metal material coating process, coating, liquid chemical plating, etc., can solve the problems of easy generation of microcracks, interlayer defects, and loose interlayer bonding, etc., to achieve enhanced resistance Hydrogen permeability, no obvious cracks, tight combination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0033] Example 1
[0034] (1) The edge of the metal zirconium hydride substrate with Φ20mm×20mm and H / Zr atomic ratio of 1.85 is chamfered at an angle of 45°, the chamfer radius is 1.5mm, the surface is polished, and then dried with acetone ultrasonic cleaning. Coating organosilica sol coating on the surface of zirconium hydride by dipping and pulling method to control the coating thickness to be about 1 μm. The temperature is increased to 50°C, 120°C, 180°C, 250°C at a temperature rising rate of 300°C / h, and the temperature is kept at 50°C, 120°C, 180°C, and 250°C for 100 hours, and then the sample is cooled to a temperature of 100°C / h Room temperature.
[0035] (2) Seal the zirconium hydride substrate with dry organosilica sol coating in a stainless steel reaction vessel with vacuum tube and vent tube, place the reaction vessel in a temperature-controlled tubular heating furnace, and vacuum the reaction vessel to 3.0 ×10 -4 Pa; After that, 5%, 15%, 80% of CO and CO are introduc...
Example Embodiment
[0038] Example 2
[0039] (1) The edge of the zirconium hydride substrate with Φ30mm×40mm and the H / Zr atomic ratio of 2.0 is chamfered to an angle of 45°, the chamfering radius is 2mm, the surface is polished, and then dried by ultrasonic cleaning with acetone. The surface of zirconium hydride is coated with an organic silica sol coating modified by nano-alumina sol by dipping and pulling method, and the coating thickness is controlled to be about 2 μm. The temperature was increased to 120°C and 200°C at a heating rate of 50°C / h, and the temperature was kept constant at 120°C and 200°C for 1 hour, and then the sample was cooled to room temperature at a temperature drop of 300°C / h.
[0040] (2) Seal the zirconium hydride substrate with dry organosilica sol coating in a quartz reaction vessel with a vacuum tube and a vent tube, place the reaction vessel in a temperature-controlled heating furnace, and vacuum the reaction vessel until it reaches 1×10 -5 Pa; After that, CO and CO of ...
Example Embodiment
[0041] Example 3
[0042] (1) The edge of the hafnium hydride substrate with Φ20mm×50mm and the H / Hf atomic ratio of 1.75 is chamfered at an angle of 45°, the surface is polished, and then dried by ultrasonic cleaning with acetone. An organic silica sol coating modified by nano-oxidized bait sol is plated on the surface of hafnium hydride by dipping and pulling method, and the thickness of the coating is controlled to be about 3 μm. The temperature was increased to 50°C, 120°C, and 180°C at a temperature increase rate of 300°C / h, and the temperature was kept at 50°C, 120°C, and 180°C for 30 hours. Then the sample was cooled to room temperature at a temperature drop rate of 50°C / h.
[0043] (2) Seal the hafnium hydride substrate with the dried organosilica sol coating in a heat-resistant glass reaction vessel with a vacuum tube and a vent tube, place the reaction vessel in a temperature-controlled heating furnace, and vacuum the reaction vessel until it is evacuated. Vacuum to 100P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com