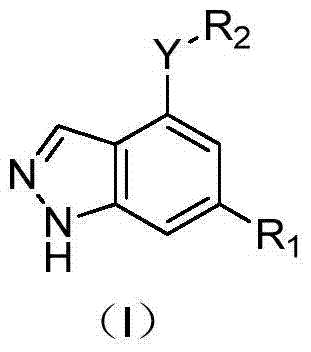
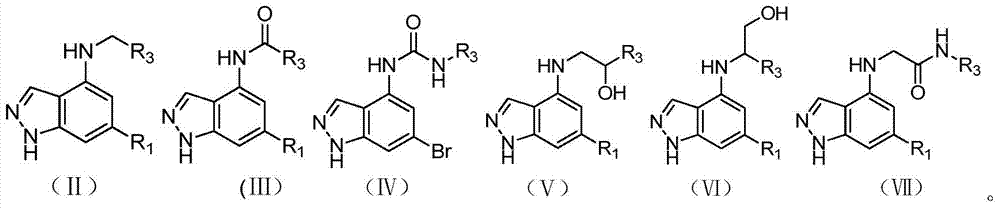
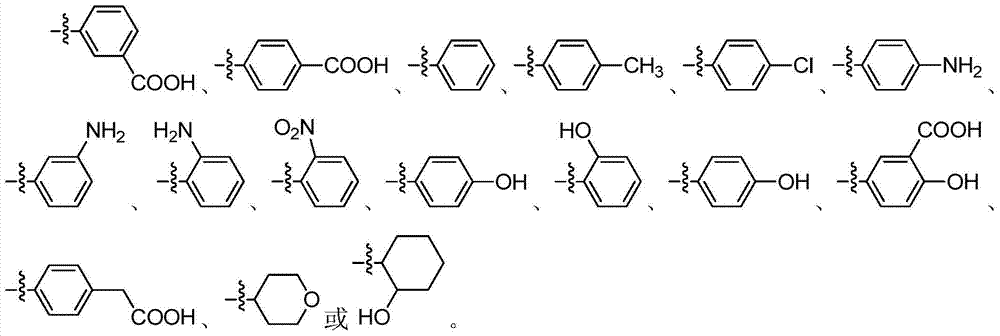
Application of 1H-indazole-4-amine compounds as IDO (indoleamine 2,3-dioxygenase) inhibitor
A compound and solvate technology, applied in the field of IDO inhibitors, can solve the problems of reducing tryptophan concentration, inhibiting killing effect, and stagnant synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The synthesis of embodiment 1 intermediate raw material
[0091] (1) Synthesis of 6a and 6b
[0092]
[0093] The raw material 4-nitro-1H-indazole 5a (CAS: 2942-40-7, 1.4g, 8.26mmol, purchased from Anaiji Chemical Reagent Co., Ltd.) or 6-bromo-4-nitro-1H-indazole Azole 5b (CAS: 885518-46-7, 2.0g, 8.26mmol, purchased from Jiangsu Nantong Biotechnology Co., Ltd.) was dissolved in a mixed solvent of ethanol (20mL) and water (10mL), and ammonium chloride (221.5mg, 4.13mmol), a part of iron powder (1.3g, 23.46mmol) was first added thereto, heated to 80°C and stirred for 5 minutes, then the remaining iron powder (1.0g, 17.86mmol) was added, and stirred for 20 minutes. After the reaction of the raw materials was detected by TLC, the reaction solution was filtered while it was hot, and the filter residue was washed with ethanol (10 mL). Ethanol was spun off under reduced pressure, and the aqueous layer was extracted three times with ethyl acetate (20 mL). The organic phas...
Embodiment 2
[0104] Synthesis of Embodiment 2 Compounds of the present invention 3a, 3b and 3c
[0105]
[0106]Synthesis of ethyl 2-(6-bromo-1H-indazole-4-amino)acetate (11)
[0107] 6-Bromo-1H-indazol-4-amino 6b (212.0 mg, 1.00 mmol) was dissolved in DMF (5 mL), and potassium carbonate (345.0 mg, 2.50 mmol) and potassium iodide (14.9 mg, 0.09 mmol) were added. Under the protection of argon, ethyl bromoacetate (167.0 μL, 1.50 mmol) was added and reacted at 65° C. overnight. After the complete reaction of the raw material (6b) was detected by TLC, a large amount of DMF was pumped away with an oil pump, ethyl acetate (25 mL) was added, and washed with water (20 mL) three times. The ethyl acetate layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and spin-dried to pass through the column (PE:EA=15:1). 206.9mg of light yellow solid was obtained, the yield was 70%.
[0108] Synthesis of 2-(6-bromo-1H-indazole-4-amino)acetic acid (12)
[0109] Dissolve ethyl 2...
Embodiment 3
[0118] Embodiment 3 Synthesis of compounds of the present invention 1a, 1c-j and 1p
[0119]
[0120] Amine 6b (0.28mmol) and benzaldehyde 16 (0.24mmol) were dissolved in dichloromethane (DCM, 3mL), dihydropyridine (DHP, 83.5mg, 0.33mmol) and appropriate amount of 4A molecular sieves (840.2mg) were added dropwise Add trifluoroacetic acid (TFA, 17.6 μL, 0.24 mmol), reflux at 40°C for 12 hours, filter the reaction solution, spin dry, and pass through the column to obtain compound 1a.
[0121] 4-((6-Bromo-1H-indazol-4-amino)methyl)benzoic acid (1a). Yield 64%; brown solid; 1 H-NMR (400MHz, d 6 -DMSO,ppm):δ12.80(br,2H,COOH and indazole-NH),8.22(s,1H,indazole-H3),7.91(d,2H,J=8.3Hz,Ar-H2and Ar-H6) ,7.49(d,2H,J=8.3Hz,Ar-H3and Ar-H5),6.64(s,1H,indazole-H7),6.01(s,1H,indazole-H5),4.53(s,2H,benzyl- CH 2 ). 13 C-NMR (100MHz, d 6 -DMSO, ppm): δ167.7, 145.3, 143.0, 142.2, 132.5, 130.0, 129.9, 127.5, 121.7, 112.6, 101.2, 100.6, 46.2. ESI-MS: 344.0035[M-H].
[0122] Select the corr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com