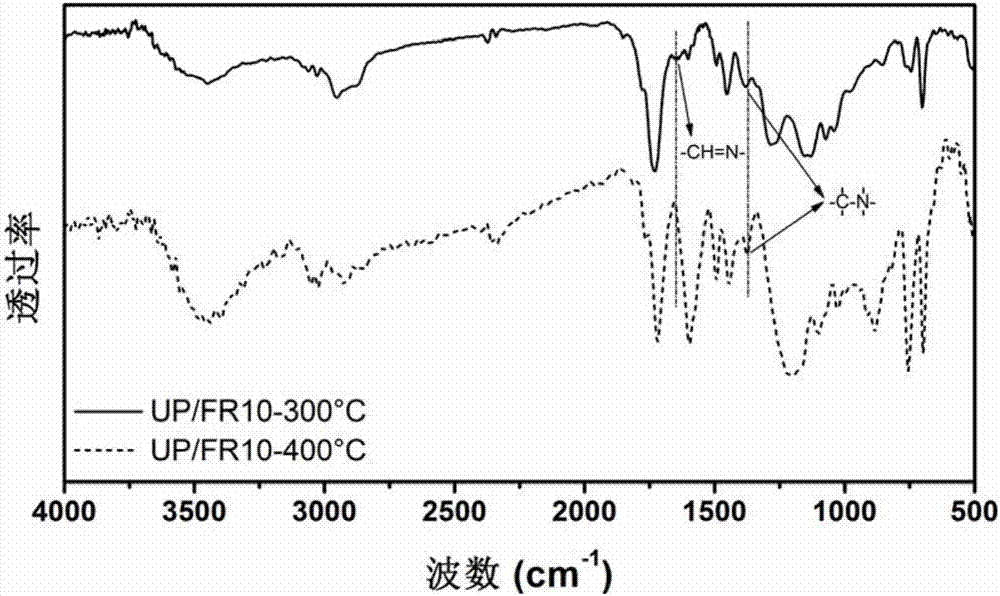
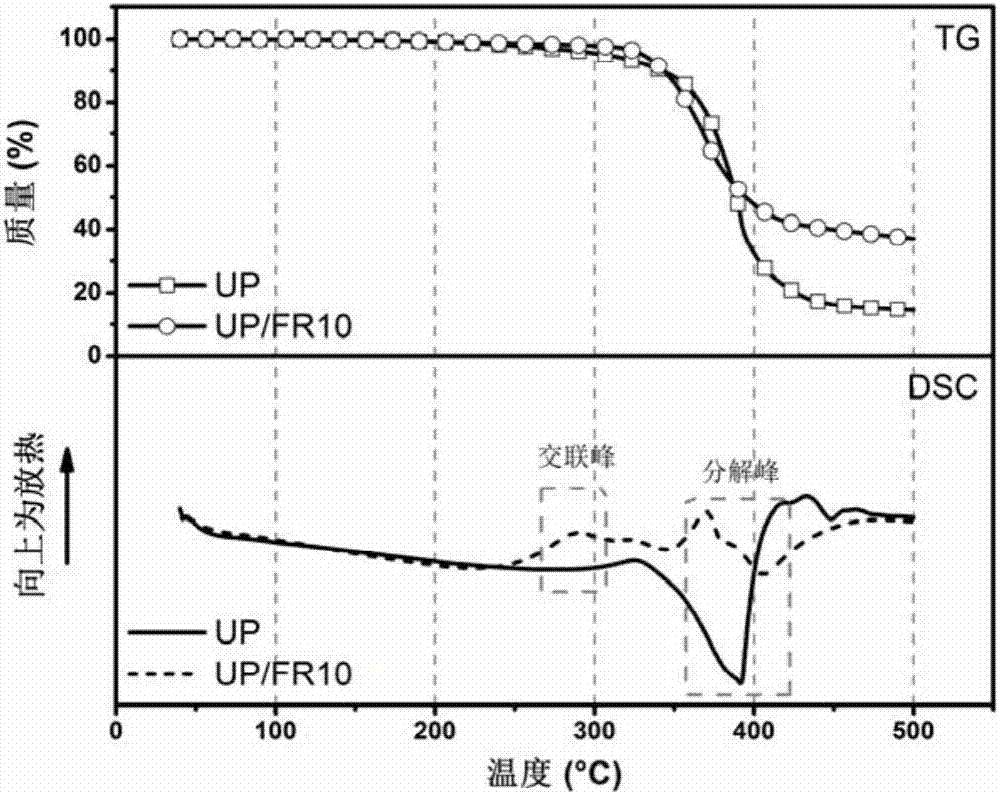
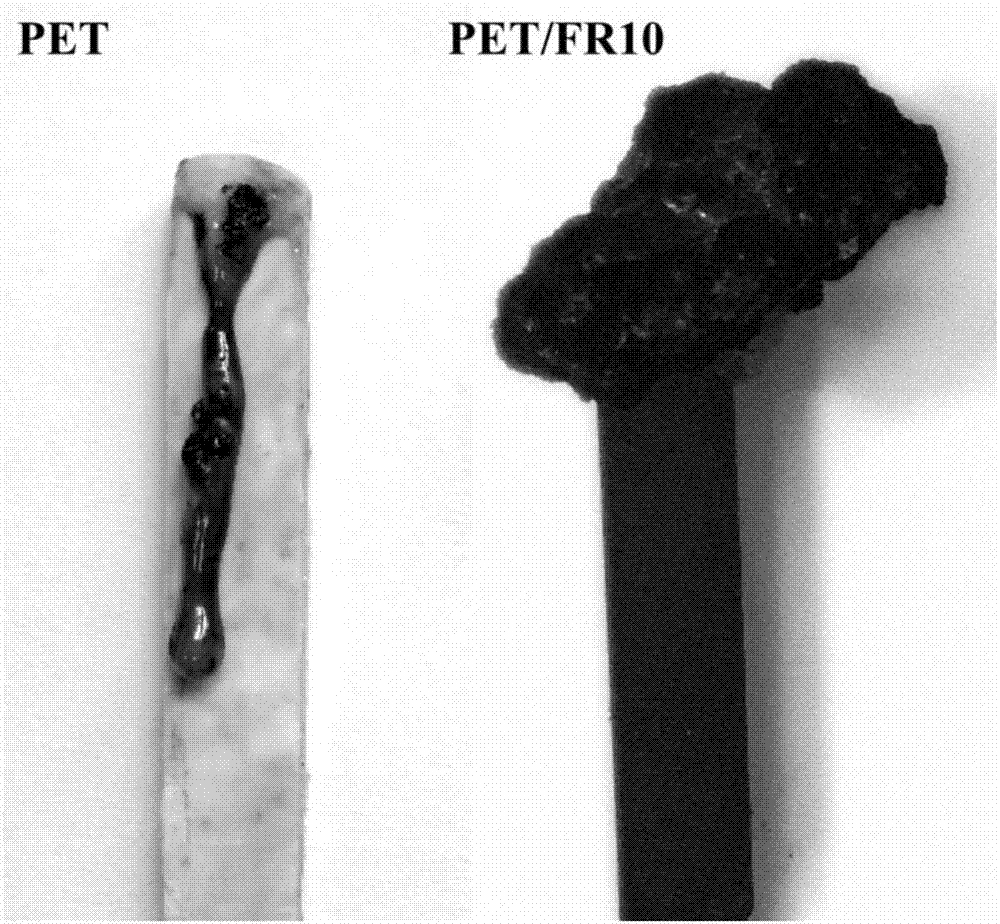
High temperature crosslinked flame retardant with Schiff base and phosphoryl (phosphonyl) structure, preparation method and application thereof
A technology of Schiff base and flame retardant, which is applied in the field of high-temperature cross-linking flame retardants and their preparation, can solve the problems of inability to cross-link, high molecular structure rigidity, easy aging and decomposition, etc., and achieve excellent flame retardancy and droplet resistance , reduce the concentration of free radicals, increase the effect of melt viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] What this embodiment prepares is flame retardant FR1, and its reaction equation is as follows:
[0042]
[0043] Heat 30.53g of p-hydroxybenzaldehyde, 18.32g of ethanolamine, and 100mL of absolute ethanol, and reflux. As the reaction progresses, yellow precipitates gradually appear in the solution. After about 6 hours, the raw materials are basically reacted completely. Suction filtration, washing with ethanol, and drying to a shallow The yellow solid was 35.49 g, the yield was 86%, and it was recorded as monomer Ⅰ-1.
[0044] Mix 35.49g of monomer Ⅰ-1 and 63.80g of monomer Ⅱ-1, under the protection of nitrogen, stir and raise the temperature to 180°C to soften and melt the system, continue to maintain the temperature until the rod climbing phenomenon occurs, and then raise the temperature to 250°C to continue the reaction 1 h gave the product as a dark yellow solid. The hydrogen chloride gas discharged from the system is absorbed with sodium hydroxide solution. Af...
Embodiment 2
[0046] What this embodiment prepares is flame retardant FR2, and its reaction equation is as follows:
[0047]
[0048] Heat 26.04g of 3-mercaptobutyraldehyde, 15.02g of ethylenediamine and 100mL of absolute ethanol, and reflux. As the reaction progresses, a yellow precipitate gradually appears in the solution. After about 8 hours, the raw materials are basically reacted completely. Suction filtration, washing with ethanol, After drying, 29.25 g of a light yellow solid was obtained, with a yield of 80%, which was designated as monomer Ⅰ-2.
[0049] Mix 29.25g of monomer I-2, 53.99g of monomer II-1 and 2.02g of calcium chloride, under the protection of nitrogen, stir and raise the temperature to 110°C to soften and melt the system, continue to maintain the temperature until the rod climbing phenomenon occurs, and then The temperature was raised to 150° C. to continue the reaction for 5 h to obtain a dark yellow solid product. The hydrogen chloride gas discharged from the sy...
Embodiment 3
[0051] What this embodiment prepares is flame retardant FR3, and its reaction equation is as follows:
[0052]
[0053] Heat 29.04g of 2-methyl-3-methylpentanal, 22.73g of p-hydroxyaniline and 100mL of absolute ethanol, and reflux. As the reaction proceeds, a yellow precipitate gradually appears in the solution. After about 6 hours, the basic reaction of the raw materials is basically complete. Filter, wash with ethanol, and dry to obtain 37.57 g of a light yellow solid with a yield of 87%, which is designated as monomer Ⅰ-3.
[0054] Mix 37.57g of monomer I-3, 51.26g of monomer II-1 and 0.02g of aluminum chloride under the protection of nitrogen, stir and raise the temperature to 170°C to soften and melt the system, continue to maintain the temperature until the rod climbing phenomenon occurs, and then The temperature was raised to 220° C. to continue the reaction for 2 h to obtain a dark yellow solid product. The hydrogen chloride gas discharged from the system is absorb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com