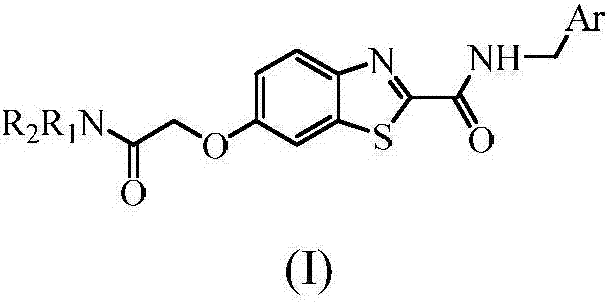
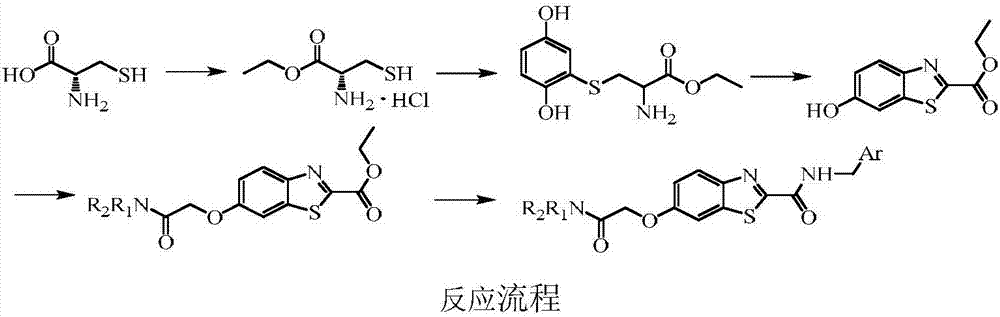
Benzothiazole formamide compound and application thereof
A compound, formamide technology, applied in the field of medicine, can solve serious problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of N-benzyl-6-(2-diethylamino-2-oxoethoxy)benzo[d]thiazole-2-carboxamide (compound ZL01)
[0035] Step A: Synthesis of L-cysteine ethyl ester hydrochloride
[0036] Put L-cysteine (24.20g, 0.20mol) and 300mL of ethanol in a 500mL eggplant-shaped flask, and slowly add thionyl chloride (22mL, 0.30mol) dropwise in an ice-water bath. Continue to react for 3h, and heat to reflux for 12h. The reaction solution was cooled to room temperature, and the solvent was evaporated to obtain 36.42 g of white powder, with a yield of 98.1%.
[0037] Step B: Synthesis of S-(2,5-dihydroxyphenyl)cysteine ethyl ester
[0038] Put L-cysteine ethyl ester hydrochloride (22.28g, 0.12mol) and methanol 60mL in a 1000mL eggplant-shaped flask, stir to dissolve it. A solution prepared by p-benzoquinone (6.48 g, 0.060 mol) and 150 mL of methanol was added dropwise in an ice-water bath. After the addition was completed, the reaction was continued at room temperature for 3...
Embodiment 2
[0057] Example 2: Synthesis of N-benzyl-6-[2-(1-piperidinyl)-2-oxoethoxy]benzo[d]thiazole-2-carboxamide (compound ZL02)
[0058] Referring to the synthesis method of Example 1, 0.92 g of pink solid was obtained, yield: 74.8%. M.p.: 198-199℃; IR: (KBr,cm -1 ):υ3429.8, 2921.8, 2852.9, 1649.8, 1521.6, 1495.8, 1384.4, 1249.8, 1216.9, 1122.7, 1085.8, 956.0, 829.1, 730.0, 694.4; ESI-MS, m / z: calcd.409.15(M + ); found 410.1([M+H] + ); 1 H NMR (400MHz, CDCl 3 ): δ7.91(d, J=9.0Hz, 1H), 7.45(d, J=2.5Hz, 1H), 7.40–7.34(m, 5H), 7.21(dd, J=9.0, 2.5Hz, 1H) ,4.78(s,2H),4.68(d,J=6.0Hz,2H),3.59(t,J=5.1Hz,2H),3.48(t,J=5.1Hz,2H),1.68–1.55(m, 6H).
Embodiment 3
[0059] Example 3: Synthesis of N-benzyl-6-[2-(4-morpholinyl)-2-oxoethoxy]benzo[d]thiazole-2-carboxamide (compound ZL03)
[0060] Referring to the synthesis method of Example 1, 0.77 g of white solid was obtained, yield: 62.6%. M.p.:186-188℃;IR:(KBr,cm -1 ):υ3421.4, 3277.0, 2923.5, 2845.7, 1666.0, 1637.9, 1496.5, 1436.6, 1359.1, 1253.8, 1210.2, 1114.7, 1001.5, 838.4, 827.6, 697.5; ESI-MS, m / z1: calcd.13 ( + ); found 412.1([M+H] + ); 1 H NMR (400MHz, CDCl 3 ): δ7.92(d,J=9.0Hz,1H),7.45(d,J=2.3Hz,1H),7.38–7.34(m,5H),7.19(dd,J=9.0,2.4Hz,1H) , 4.79 (s, 2H), 4.68 (d, J=6.0Hz, 2H), 3.67–3.65 (m, 8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com