Crystalline morphology of Iguratimod intermediate VI
A technology of crystal form and intermediate, applied in the field of crystal form and preparation of Ailamod intermediate VI
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]In a 50mL four-necked reaction flask, add 25mL of acetonitrile, 5.27g of aluminum trichloride, 7.4g of Iguratimod intermediate V (α-formamidomethyl-2-methoxy-4-methanesulfonyl- 5-phenoxyacetophenone) and 4.72g sodium iodide, reacted at 50°C for 5h, lowered the temperature, added 74g of 1% (mass percentage) sodium sulfite solution, filtered, washed with water, and drained to obtain 7.0g of the product, with a weight yield of 94.59 %. The crystal form 1 of the pure iguratimod intermediate VI was obtained by refining with methanol, with a purity of 99.8% and a melting point of 172.6-173.7°C.
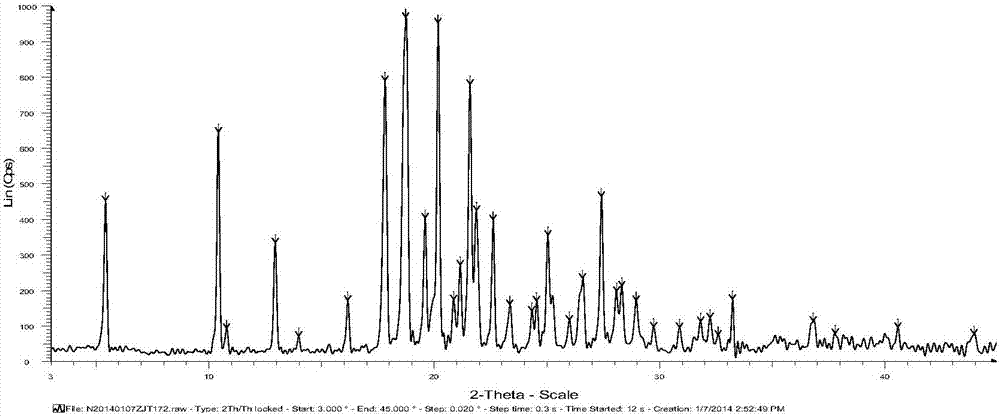
[0057] The XRD of Form 1 of Iguratimod Intermediate VI is as follows: figure 1 As shown, the graph shows that the 2θ angle has characteristic peaks at the following positions: about 5.373°, about 10.398°, about 12.923°, about 17.803°, about 18.721°, about 19.586°, about 20.160°, about 21.584°, about 21.876° , about 22.601°, about 25.048°, about 27.421°. Its differential thermal ana...
Embodiment 2
[0059] In a 50mL four-necked reaction flask, add 25mL of acetonitrile, 5.27g of aluminum trichloride, 7.4g of Ilamomod intermediate V (α-formamidomethyl-2-methoxy-4-methanesulfonamido- 5-phenoxyacetophenone) and 4.72g sodium iodide, reacted at 50°C for 5h, lowered the temperature, added 74g of 1% (mass percentage) sodium sulfite solution, filtered, washed with water, and drained to obtain 6.92g of the product, with a weight yield of 93.51 %. Purified with acetonitrile to obtain the crystalline form 2 of the pure iguratimod intermediate VI with a purity of 99.7% and a melting point of 191.0-192.3°C.
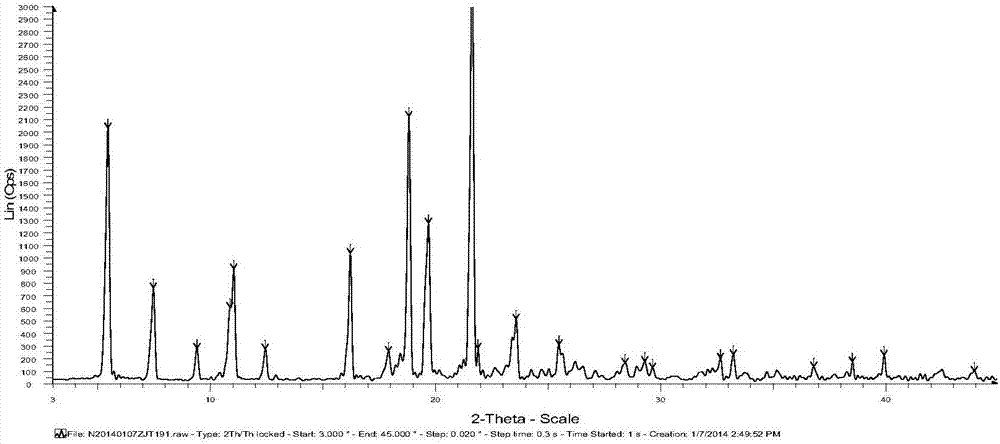
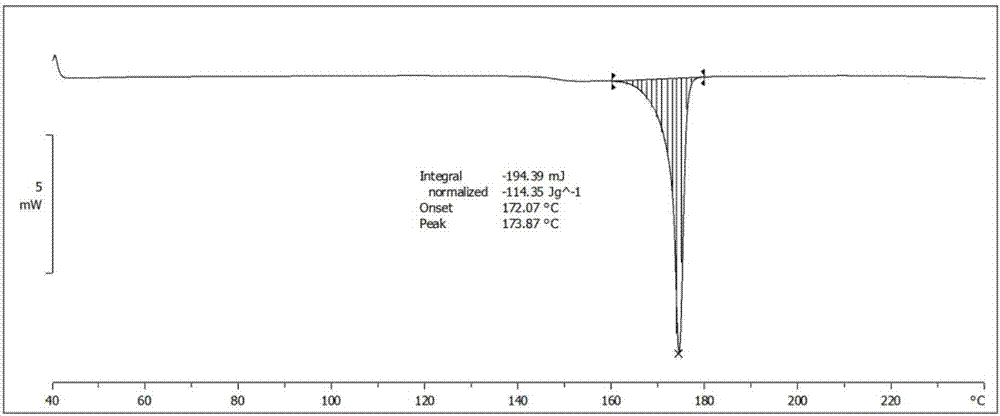
[0060] The XRD of Form 2 of Iguratimod Intermediate VI is as follows: figure 2 As shown, the spectrum shows that the 2θ angle has characteristic peaks at the following positions: about 5.408°, about 7.432°, about 11.006°, about 16.201°, about 18.808°, about 19.675°, about 21.621°. Its differential thermal analysis DSC diagram is as follows Figure 4 As shown, their endothermic...
Embodiment 3
[0062] Add 15mL of acetonitrile, 5.27g of aluminum trichloride, 7.4g of Alamod intermediate V (α-formamidomethyl-2-methoxy-4-methanesulfonamido-5 -phenoxyacetophenone) and 4.72g of sodium iodide were reacted at 50°C for 5h, cooled, and 74g of 1% sodium sulfite solution was added, filtered, washed with water, and drained to obtain 6.81g of the product, with a weight yield of 92.0%. Purified with methanol to obtain the crystalline form 1 of the pure iguratimod intermediate VI with a purity of 99.0% and a melting point of 172.2-173.3°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com