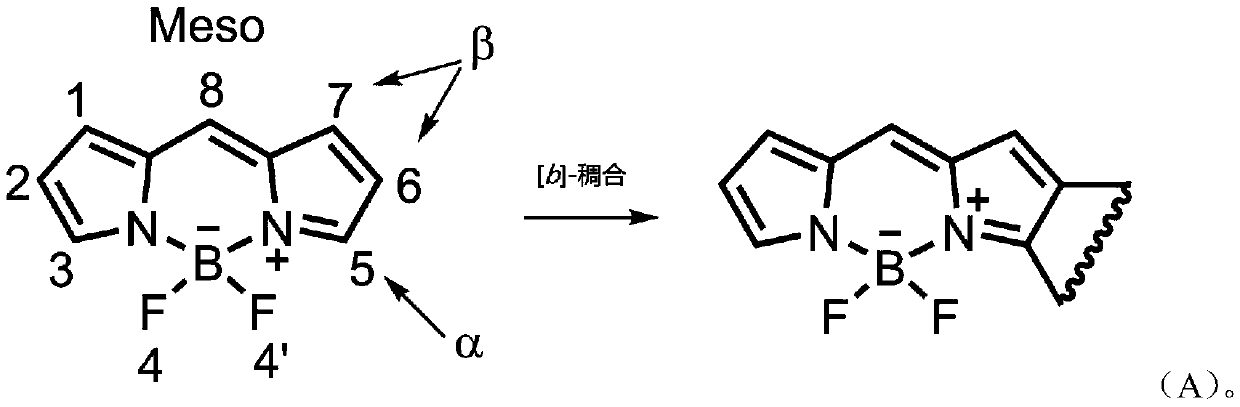
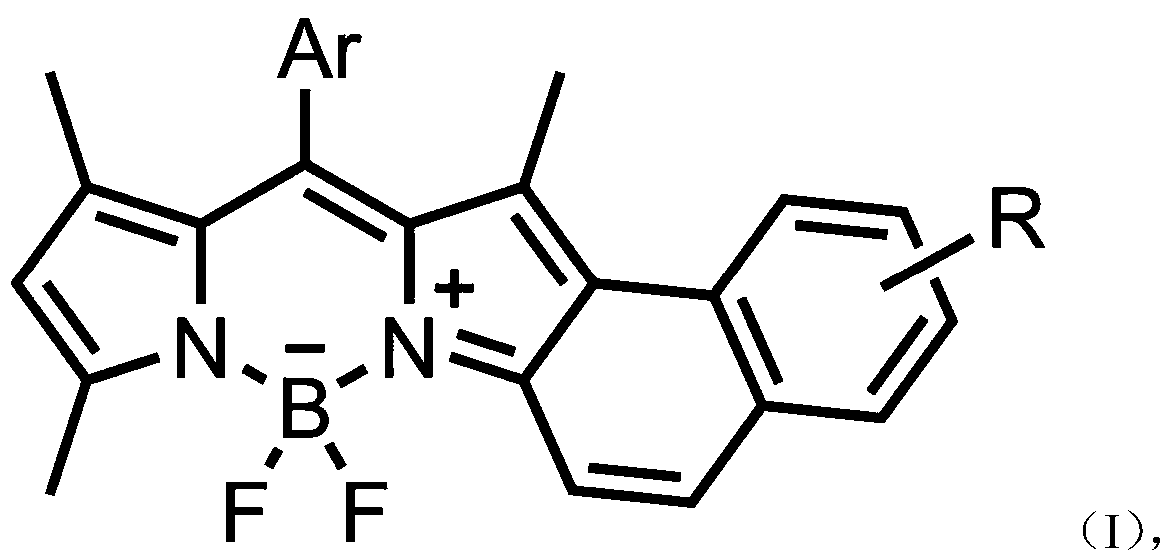
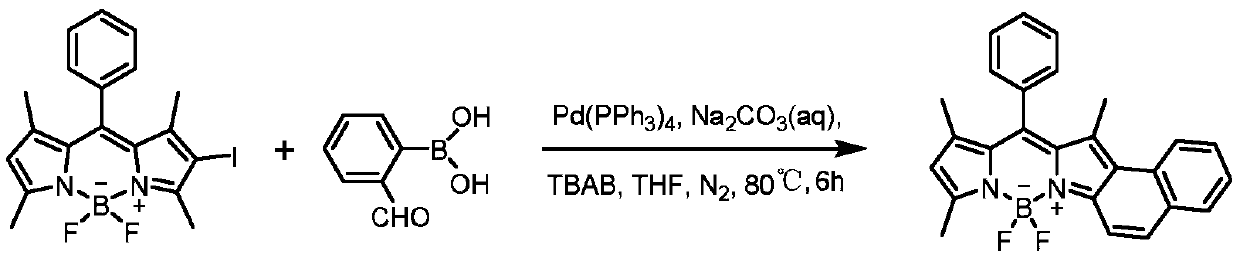Asymmetric naphthalene ring fused fluoroboron dipyrrole fluorescent dye and preparation method thereof
A technology of naphthalene ring fused fluoroboron dipyrrole and fluoroboron dipyrrole, which is applied in the field of asymmetric naphthalene ring fused fluoroboron dipyrrole fluorescent dyes and its preparation, which can solve the problems of cumbersome synthesis methods and achieve simple preparation methods , high yield, improved light stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 50ml Schlenk reaction flask, add monoiodo BODIPY (50mg, 0.11mmol), 2-formylphenylboronic acid (25mg, 0.165mmol), tetrakistriphenylphosphine palladium (13mg, 0.011mmol), 20ml tetrahydrofuran, TBAB (35mg, 0.11mmol), 3mL 2M Na 2 CO 3 aqueous solution. Nitrogen was blown for 20 minutes, and the reaction was stirred at 80°C for 6 hours. Separation with a chromatographic column (DCM:PE=35:65) gave 17.7 mg of a purple-black solid product with a yield of 39%. The product whose structural formula is shown in (I-1) is an asymmetric naphthalene ring-fused fluorobodipyrrole fluorescent dye. 1 H NMR (400MHz, CDCl 3 )δ=8.22(d,1H),7.88(d,1H),7.80(d,1H),7.69(d,1H),7.57–7.56(m,3H),7.48(t,1H),7.41–7.37 (m,3H),6.16(s,1H),2.69(s,3H),2.01(s,3H),1.45(s,3H)ppm.MALDI-TOF-MS:calcd for[C 26 h 21 BF 2 N 2 ] + :410.18; found: 410.57[M] + ,391.52[M-F] + .
[0024] The synthetic reaction equation is as follows:
[0025]
[0026]
Embodiment 2
[0028] In a 50ml Schlenk reaction flask, add monoiodo BODIPY (50mg, 0.11mmol), 5-fluoro-2-formylphenylboronic acid (28mg, 0.165mmol), tetrakistriphenylphosphine palladium (13mg, 0.011mmol), 20ml THF, TBAB (35mg, 0.11mmol), 3mL 2M Na 2 CO 3 aqueous solution. Nitrogen was blown for 20 minutes, and the reaction was stirred at 80°C for 6 hours. Separation with a chromatographic column (DCM:PE=35:65) can obtain 21 mg of a purple-black solid product with a yield of 45%. The product of the structural formula (I-2) is an asymmetric naphthalene ring-fused fluorobodipyrrole fluorescent dye.
[0029] 1 H NMR (400MHz, CDCl 3 )δ=7.88-7.77(m,3H),7.67(d,1H),7.59–7.56(m,3H),7.41-7.38(m,2H),7.16-7.11(m,1H),6.18(s, 1H), 2.70(s,3H), 1.97(s,3H), 1.46(s,3H)ppm.
[0030] MALDI-TOF-MS: calcd for [C 26 h 20 BF 3 N 2 ] + :428.17; found: 428.47[M] + ,409.58[M-F] + .
[0031] The synthetic reaction equation is as follows:
[0032]
Embodiment 3
[0034] In a 50ml Schlenk reaction flask, add monoiodo BODIPY (50mg, 0.11mmol), 3-fluoro-2-formylphenylboronic acid (28mg, 0.165mmol), tetrakistriphenylphosphine palladium (13mg, 0.011mmol), 20ml THF, TBAB (35mg, 0.11mmol), 3mL 2M Na 2 CO 3 aqueous solution. Nitrogen was blown for 20 minutes, and the reaction was stirred at 80°C for 6 hours. Separation with a chromatographic column (DCM:PE=35:65) gave 10 mg of a purple-black solid product with a yield of 21%. The asymmetric naphthalene ring-fused fluorobodipyrrole fluorescent dye with the structural formula (I-3).
[0035] 1 H NMR (400MHz, CDCl 3)δ=8.03-8.01(m,3H),7.95(d,1H),7.72–7.67(m,1H),7.58(t,3H),7.42-7.37(m,2H),7.08(t,1H) ,6.19(s,1H),2.71(s,3H),1.99(s,3H),1.46(s,3H)ppm.
[0036] MALDI-TOF-MS: calcd for [C 26 h 20 BF 3 N 2 ] + :428.17; found: 428.34[M] + ,409.25[M-F] + .
[0037] The synthetic reaction equation is as follows:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com