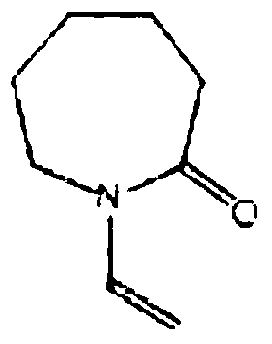
A kind of preparation method of vinyl caprolactam
A technology of vinyl caprolactam and base caprolactam, which is applied in the preparation of vinyl caprolactam and the field of crystal products, can solve the problems of easy correlation reaction, high technical difficulty, affecting product yield, etc., and solve the problems of catalyst decomposition and deactivation, The effect of simplifying the preparation process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
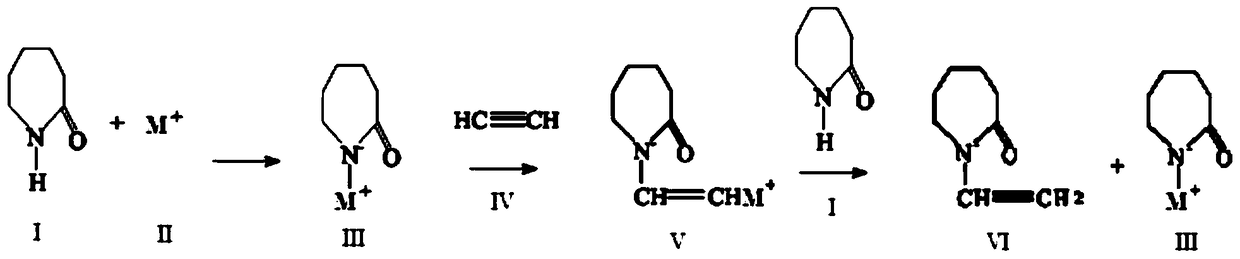
[0058] In a 3000ml four-necked bottle equipped with an electric stirrer, a vacuum distillation device, a thermometer, and a vent valve, add 400g of caprolactam and 12g of potassium hydroxide for vacuum distillation, heat and melt for dehydration, the dehydration temperature is 80-150°C, and the time is 30- 90min, vacuum degree -0.01 to -0.085MPa, until no liquid drops flow out, forming a nucleophilic addition reaction system.
[0059] Continuously feed acetylene into the above system to carry out vinylation addition reaction. The set reaction temperature is 120°C, and the set temperature change is controlled in the reactor so that the set temperature change is controlled between 120±5°C and the pressure is 0-0.01MPa. The ventilation volume of acetylene is 0.0786m 3 , the ventilation rate is 160ml / min. When the ventilation is continued for 20 minutes, the temperature of the system continues to drop below 90°C, and the pressure of the system continues to rise above 0.01MPa, it...
Embodiment 2
[0063] Add 500g of caprolactam and 15g of potassium hydroxide to a 3000ml four-necked bottle equipped with an electric stirrer, a vacuum distillation device, a thermometer, and a vent valve, and heat and melt for dehydration. The dehydration temperature is 100-150°C and the time is 60-90min. , Vacuum degree -0.085 to -0.001MPa, until no liquid drops flow out, forming a nucleophilic addition reaction system.
[0064] Continuously feed acetylene into the above system to carry out vinylation addition reaction. The set reaction temperature is 100°C, and the set temperature change is controlled in the reactor so that the set temperature change is controlled between 100±5°C and the pressure is 0-0.025MPa. The ventilation volume of acetylene is 0.0983m 3 , the ventilation rate is 102ml / min. When the ventilation is continued for 25 minutes, the temperature of the system continues to drop below 90°C, and the pressure of the system continues to rise above 0.025 MPa, it is the end of t...
Embodiment 3
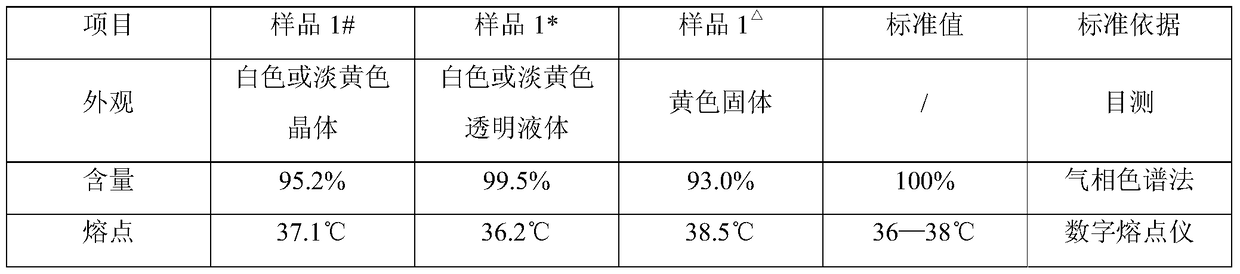
[0072] Preliminary purification product sample 1#, final purification product sample 1* and vinyl caprolactam sample 1 in comparative example 1 in Example 1 △ , to conduct relevant performance tests. The specific data results are shown in Table 1.
[0073] Table 1 Performance test results comparison table
[0074]
[0075] It can be seen from Table 1 that the product purity of the preliminary purified product sample and the final purified product sample prepared in Example 1 are ≥95% and ≥99% respectively, which are better than the vinylcaprolactam sample prepared in Comparative Example 1. At the same time, the final purified product sample prepared in Example 1 is a white or light yellow transparent liquid. Compared with the solid sample prepared in Comparative Example 1, no block crystals are formed, which is more convenient for packaging, storage, and use. Moreover, the sample prepared in Example 1 has a lower melting point than Comparative Example 1, which can meet th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com