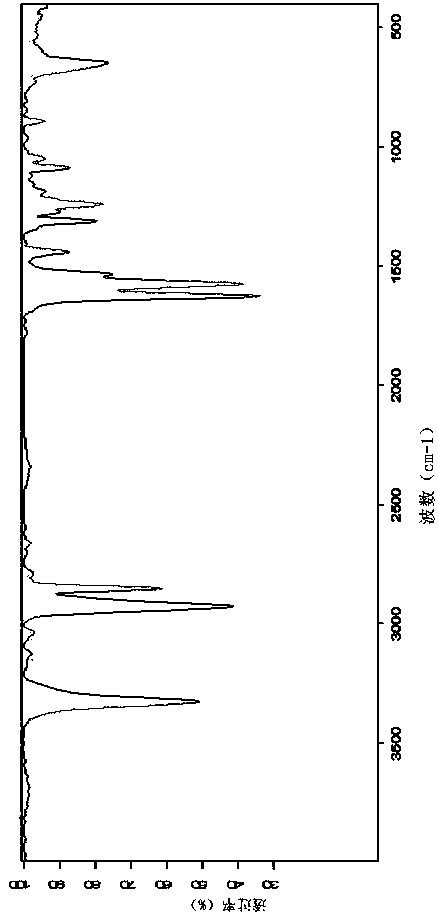
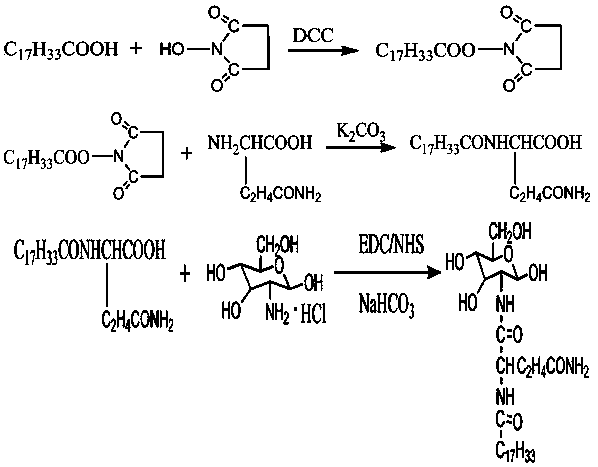
n-(n'-oleoyl glutaminyl)-glucosamine and its preparation method
A technology of oleoylglutamyl and glucosamine, which is applied in the field of surfactant preparation, can solve rare problems, achieve mild properties, good biocompatibility, and save reaction time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] (1) Dissolve 0.282g (1.0mmol) oleic acid in anhydrous DMF first, add 0.12g (1.05mmol) NHS (N-hydroxysuccinimide) and 0.226g (1.1mmol) dicyclohexylcarbodiethylene Amine (DCC), stirred and reacted for 10 to 12 hours, then filtered to obtain filtrate (I). Then take 0.146g (1mmol) of glutamine and 0.138g (1mmol) of potassium carbonate and dissolve them in an appropriate amount of water, place them in an ice-water bath, slowly add the filtrate (I) dropwise, and after the dropwise addition, place it at room temperature for 24 hours to react. A solution of N-acyl amino acids is prepared.
[0024] (2) Adjust the pH of N-oleoyl glutamine solution to neutral with 3N hydrochloric acid, then add 0.216g glucosamine hydrochloride, 0.84g sodium bicarbonate, 0.203g EDAC (1-(3-dimethylaminopropyl )-3-ethylcarbodiimide hydrochloride), reacted at 0°C to room temperature for 24 hours, and centrifuged to obtain crude N-(N′-oleoyl glutaminyl)-glucosamine.
[0025] (3) Dissolve crude N-(N′-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com