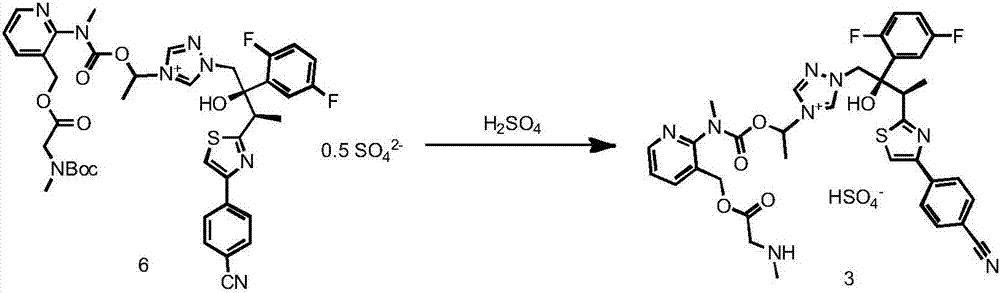
Preparation method of isavuconazonium monosulfate through oxidation-reduction reactions
A technology of isavuconazolium and sulfate, which is applied in the field of pharmacy, can solve problems such as instability, difficulty in industrial production, and poor operability of the process, and achieve simple process operation, precise ratio control, and avoid process steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation technology of formula 4 compound is:
[0037]
[0038] (1) preparation of formula 8 compounds
[0039] In the 5L three-necked round bottom reaction flask, add formula 10 compound successively, i.e. diethyl dithiophosphate (804g), formula 7 compound (300g), Virahol (1.5L) and purified water (194ml), heat up under stirring To reflux reaction (oil temperature 85°C), internal temperature 78°C, keep reflux, gradually a large amount of H 2 S gas is released until the temperature stabilizes at an internal temperature of 78°C, without a large amount of gas being released, and the temperature is kept stirring. After 15 hours, sampling and monitoring were performed, and TLC showed that SM1 spots disappeared and new spots were formed.
[0040] Cool down to below 20°C, add DCM (1.5L), purified water (1.5L). Slowly add 10wt% NaOH solution (about 2.3 L) dropwise under stirring to adjust the pH value to 7-8, and the system turns yellow and gray during the additio...
Embodiment 1
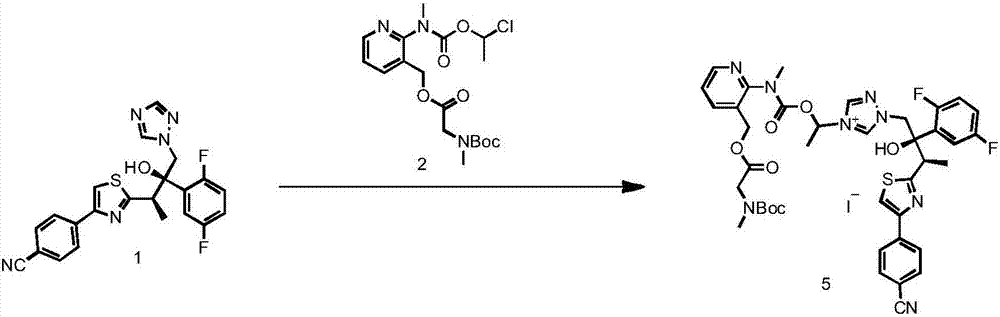
[0055] The preparation of formula 5 compound
[0056] The compound of formula 4 (100 g), the compound of formula 2 (133 g), NaI (48 g) and anhydrous acetonitrile (500 ml) were sequentially added into a 1 L single-necked round-bottomed reaction flask, replaced by nitrogen, and protected by nitrogen. Under stirring, heat up to oil temperature of 60°C, internal temperature of 50°C, keep stirring for 12 hours. Cool down to below 20°C, filter, and wash the filter cake with EA. EA (400ml) and purified water (1000ml) were added to the filtrate, followed by stirring and extraction. Separation, the upper EA phase is wine red. The lower aqueous phase was extracted twice with EA (100ml), the organic phases were combined, washed with saturated NaCl solution (100ml), dried by adding 40g of anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a foamy solid, which was directly used in the next reaction.
[0057] Yield 81%. HPLC purity >90%.
Embodiment 2
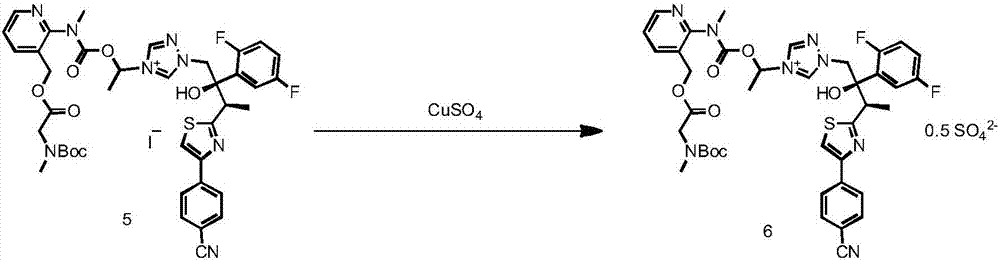
[0059] The preparation of formula 6 compound
[0060] The concentrated foamy solid in Example 1 was dissolved in 900ml of EA, then 900ml of purified water was added, and placed in a three-necked round-bottomed reaction flask of 3L, after adding CuSO 4 5H 2 O (80g), keep stirring at 25°C for 1h, the color of the system changes from deep red to wine red during the stirring process.
[0061] Leave to stand for stratification, separate the aqueous phase and the organic phase, the color of the aqueous phase is blue, and the organic phase is wine red. The organic phase was washed twice with water, washed with saturated NaCl solution, dried over anhydrous sodium sulfate (30 g), and concentrated to a foamy solid. The foamy solid was dissolved in DCM (0.5 L), and silica gel (300 g) was added, stirred evenly, and then rotary evaporated under reduced pressure to powder. The samples were loaded by dry method, purified by column chromatography, 2000g of silica gel was loaded into the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com