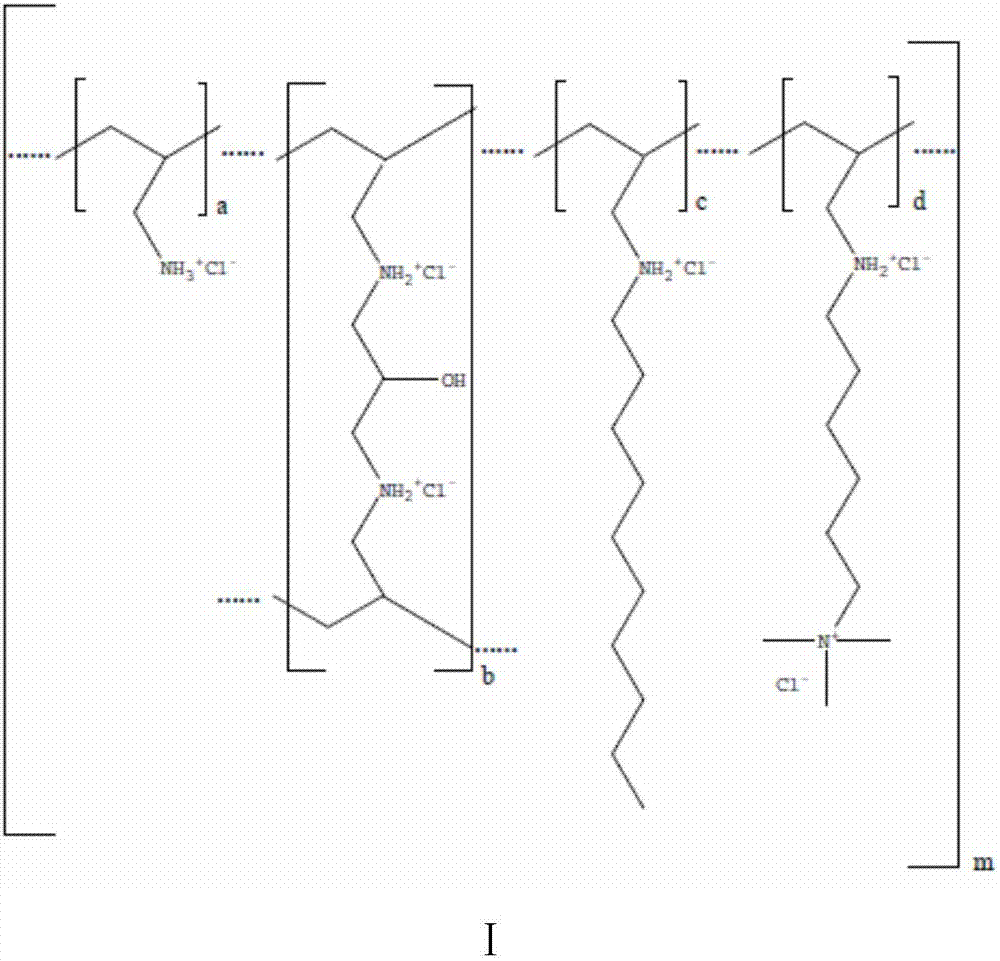
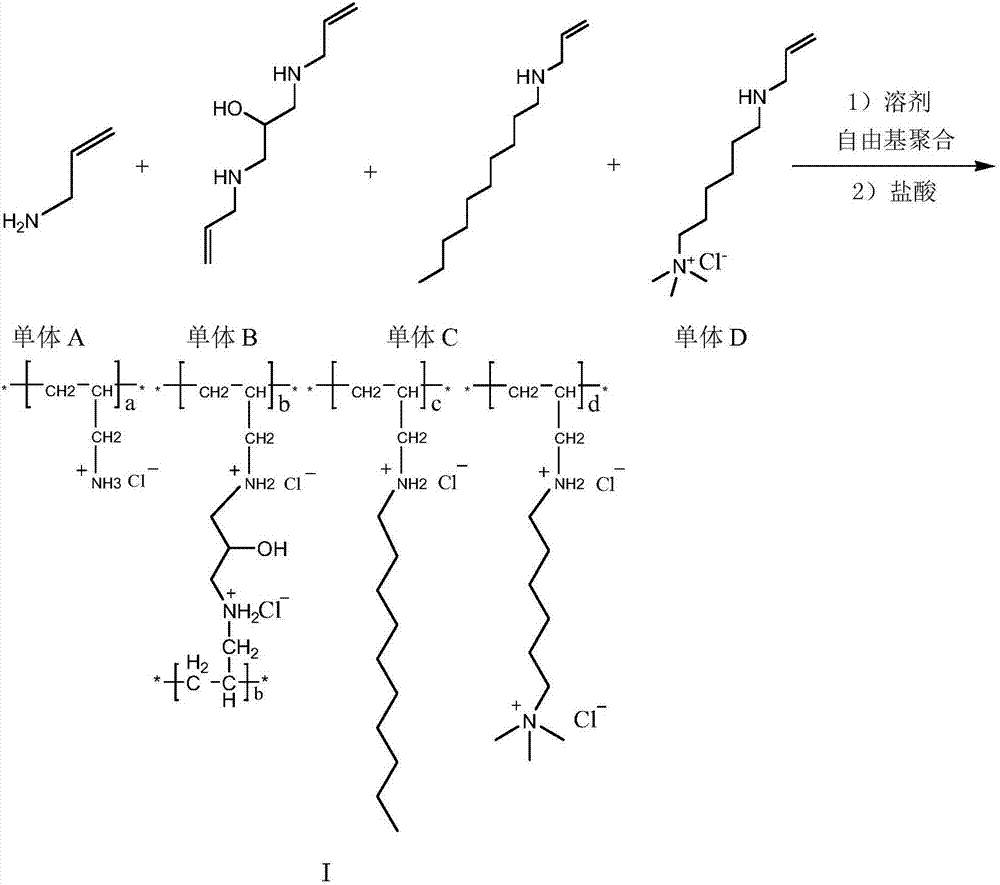
Method for preparing colesevelam hydrochloride by solution polymerization
A technology of colesevelam hydrochloride and polymerization reaction, which is applied in the field of drug synthesis, can solve the problems of not being simple and simple in operation steps and not easy to control the degree of crosslinking, and achieve the goal of easy control of grafting rate, guarantee of stability and high reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
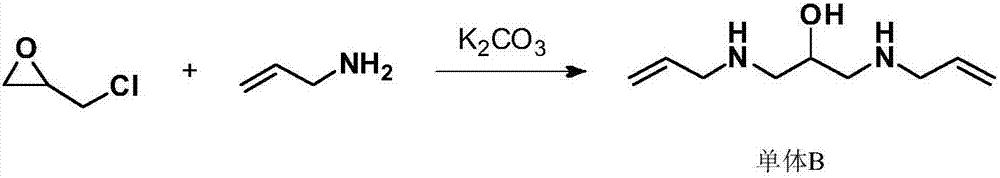
[0034] Example 1: Preparation of monomer B (1,3-diallylamino-2-hydroxypropane)
[0035]
[0036] In a four-necked flask equipped with stirring, a thermometer, a constant pressure dropping funnel, and a condensing reflux tube, add 350 grams of 1,2-dichloroethane, 57.0 grams (1.0 moles) of allylamine, 79.0 grams (0.5 moles) ) Potassium carbonate, heated and kept at 40-45°C and slowly added 46.2 grams (0.5 moles) of epichlorohydrin dropwise, and the dropwise addition was completed in about 4 hours. 20-25°C, filter, wash the filter cake with 50 g of 1,2-dichloroethane, combine the organic phases, recover 1,2-dichloroethane by atmospheric distillation, and then distill under reduced pressure to obtain a colorless transparent liquid 1,3 - Diallylamino-2-hydroxypropane 80.3 g, GC purity 99.8%, yield 94.5%.
Embodiment 2
[0037] Example 2: Preparation of monomer B (1,3-diallylamino-2-hydroxypropane)
[0038] Replace the 1,2-dichloroethane in Example 1 with tetrahydrofuran of the same quality, and the rest are the same as in Example 1 to obtain 81.1 grams of colorless and transparent liquid 1,3-diallylamino-2-hydroxypropane, with a GC purity of 99.7%, the yield is 95.3%.
Embodiment 3
[0039] Embodiment 3: the preparation of monomer C (N-allyl n-decylamine)
[0040]
[0041] In a four-necked flask equipped with stirring, a thermometer, a constant pressure dropping funnel, and a condensing reflux tube, add 550 grams of 1,2-dichloroethane, 57.0 grams (1.0 moles) of allylamine, 138.0 grams (1.0 moles) ) Potassium carbonate, heated and kept at 40-45°C, and slowly added 221.0 grams (1.0 mole) of 1-bromodecane dropwise, and the dropwise addition was completed in about 4 hours, and continued to stir and react at 60-65°C for 6 hours, and cooled to 20-25°C, filter, wash the filter cake with 100 g of 1,2-dichloroethane, combine the organic phases, recover 1,2-dichloroethane by atmospheric distillation, and then distill under reduced pressure to obtain a colorless transparent liquid N-ene Propyl n-decylamine 190.5 g, GC purity 99.5%, yield 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com