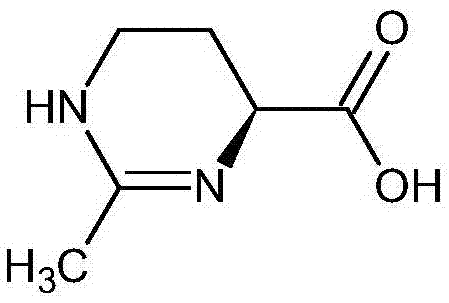
(S)-2-methyl-1,4,5,6-tetrahydromethylpyrimidine-4-carboxylic acid synthesis method
A tetrahydromethylpyrimidine and synthetic method technology, applied in the field of cyclic amino acid synthesis, can solve the problems of low environmental pollution, retention, and low yield, and achieve the effects of controllable process, stable reaction, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
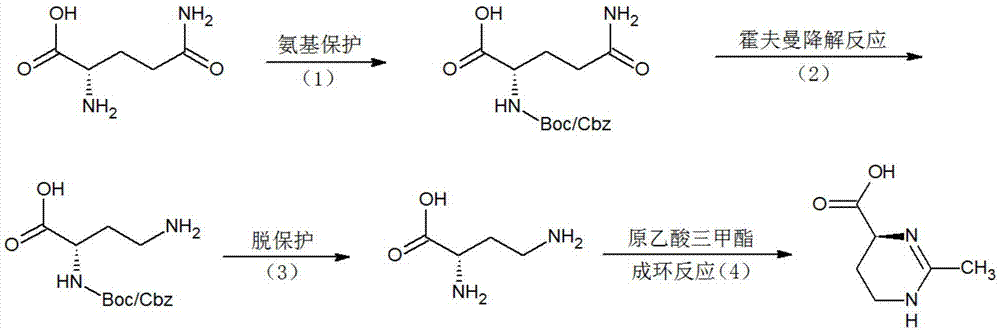
Image
Examples
Embodiment 1
[0048] Synthesis of tert-butoxycarbonyl-L-glutamine:
[0049] In the 500ml reaction flask, drop into 10 grams of glutamine, 200 milliliters of 10% sodium carbonate solution, stir and dissolve, add 100 milliliters of acetone, then add dropwise the mixed solution of 30 grams of di-tert-butyl dicarbonate and 20 milliliters of acetone at room temperature, simultaneously Constantly adjust the pH to 8-9 with sodium carbonate, and react overnight at room temperature. Adjust the pH to 2 with 3N hydrochloric acid, then extract 5 times with ethyl acetate, combine the ethyl acetate, wash with saturated brine three times, and evaporate the ethyl acetate to obtain 12 g of tert-butoxycarbonyl-L-glutamine. Melting point: 206-208°C.
Embodiment 2
[0051] Synthesis of Benzyloxycarbonyl-L-Glutamine:
[0052]Put 30 grams of glutamine and 500 milliliters of 10% sodium carbonate solution into a 1L reaction bottle, stir to dissolve, add 300 milliliters of acetone, then add 60 grams of benzyl chloroformate dropwise at room temperature, and continuously adjust the pH to 8- 9. React overnight at room temperature. Use 3N hydrochloric acid to adjust the pH to 2, then extract 5 times with ethyl acetate, combine the ethyl acetate, wash with saturated brine three times, then evaporate the ethyl acetate to obtain 50 g of benzyloxycarbonyl-L-glutamine. Melting point: 135-137°C.
Embodiment 3
[0054] Synthesis of p-toluenesulfonyl-L-glutamine:
[0055] Put 20 grams of glutamine and 200 milliliters of 10% sodium carbonate solution into a 500ml reaction bottle, stir to dissolve, add 100 milliliters of acetone, then add dropwise the mixed solution of 40 grams of p-toluenesulfonyl chloride and 50 milliliters of acetone at room temperature, while constantly using Adjust the pH to 8-9 with sodium carbonate, and react overnight at room temperature. Adjust the pH to 1 with 3N hydrochloric acid, then extract 5 times with ethyl acetate, combine the ethyl acetate, wash with saturated brine three times, and evaporate the ethyl acetate to obtain 32 g of p-toluenesulfonyl-L-glutamine. Melting point: 146-148°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com