Method for preparing herbicide flufenacet intermediate tda-sulfone
A synthetic method, thiadiazole technology, applied in the direction of organic chemistry, can solve problems such as difficult to remove, uncontrollable safety, long reaction time, etc., to increase the service life of equipment, avoid large amounts of aggregation, and improve the intrinsic safety of the process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
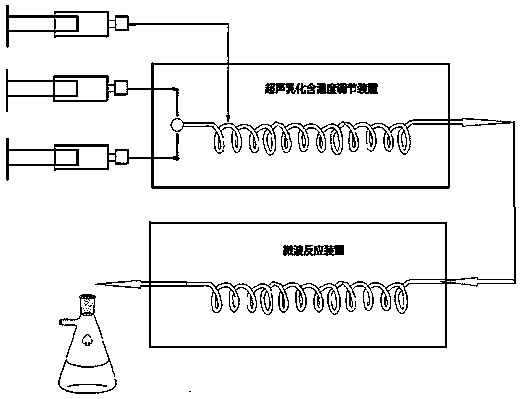
[0043] 1) The device used: using figure 2 Schematic diagram of the reaction apparatus and equipment used; and image 3 A schematic diagram of the process shown.
[0044] 2) Select toluene as a solvent, dissolve the raw material 2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole in an appropriate amount of solvent toluene to form 2-methylthio-5-trifluoromethyl The solution of base-1,3,4-thiadiazole raw material, the raw material concentration is 26%;
[0045] 3) Dissolve the catalyst phenethylphenol polyoxyethylene ether in an appropriate amount of toluene to form a toluene solution of the catalyst phenethylphenol polyoxyethylene ether, and the catalyst concentration is 5%;
[0046] 4) Parameter control: through metering pump 1 (2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole raw material), metering pump 2 (70% hydrogen peroxide), control 70% hydrogen peroxide and raw materials The molar ratio of 2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole is about 2.2:1, where metering ...
Embodiment 2
[0053] 1) The device used: using figure 2 Schematic diagram of the reaction apparatus and equipment used; and image 3 A schematic diagram of the process shown. And the Corning microchannel reactor with exactly the same improvement as in Example 1 was used.
[0054] 2) Select toluene as a solvent, dissolve the raw material 2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole in an appropriate amount of solvent toluene to form 2-methylthio-5-trifluoromethyl The solution of base-1,3,4-thiadiazole raw material, the raw material concentration is 26%;
[0055] 3) Parameter control: through metering pump 1 (2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole raw material), metering pump 2 (70% hydrogen peroxide), control 70% hydrogen peroxide and raw materials The molar ratio of 2-methylthio-5-trifluoromethyl-1,3,4-thiadiazole is about 2.2:1, where metering pump 1 (2-methylthio-5-trifluoromethyl-1 , 3,4-thiadiazole raw material) flow rate is 14.4 mL / min, and the flow rate of metering p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com