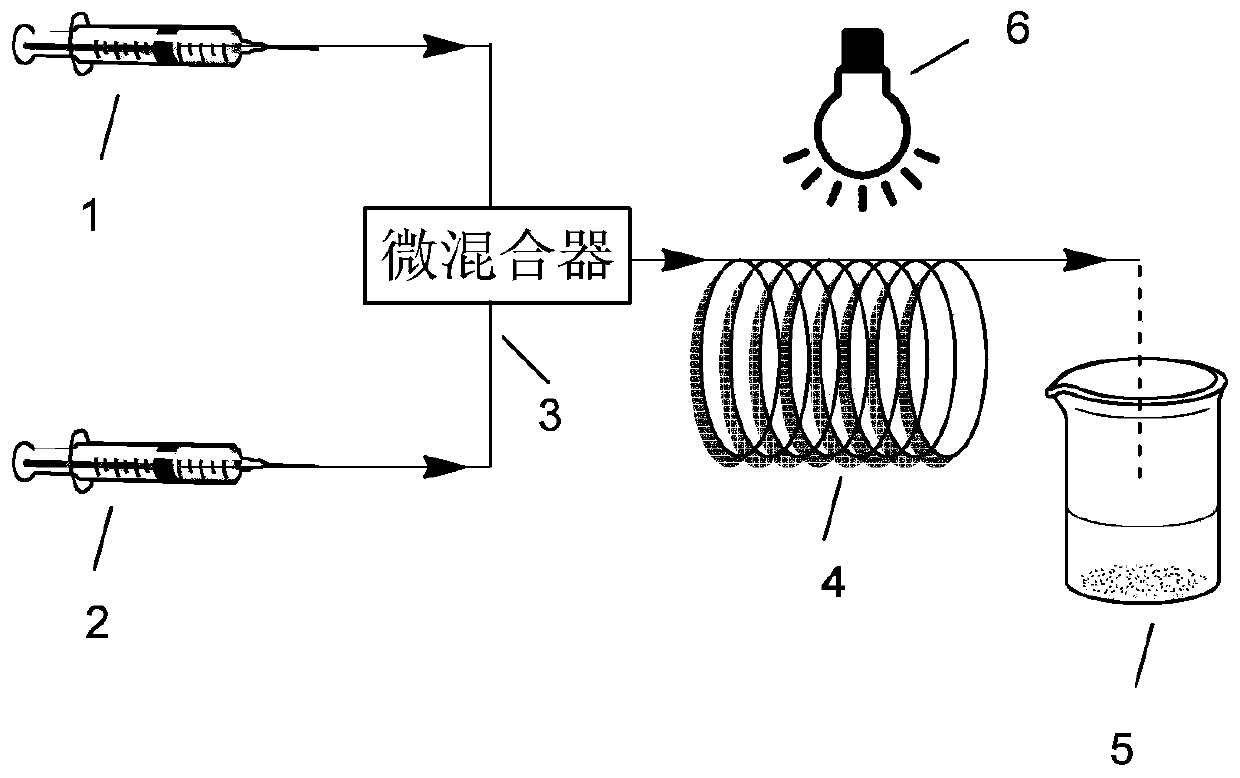
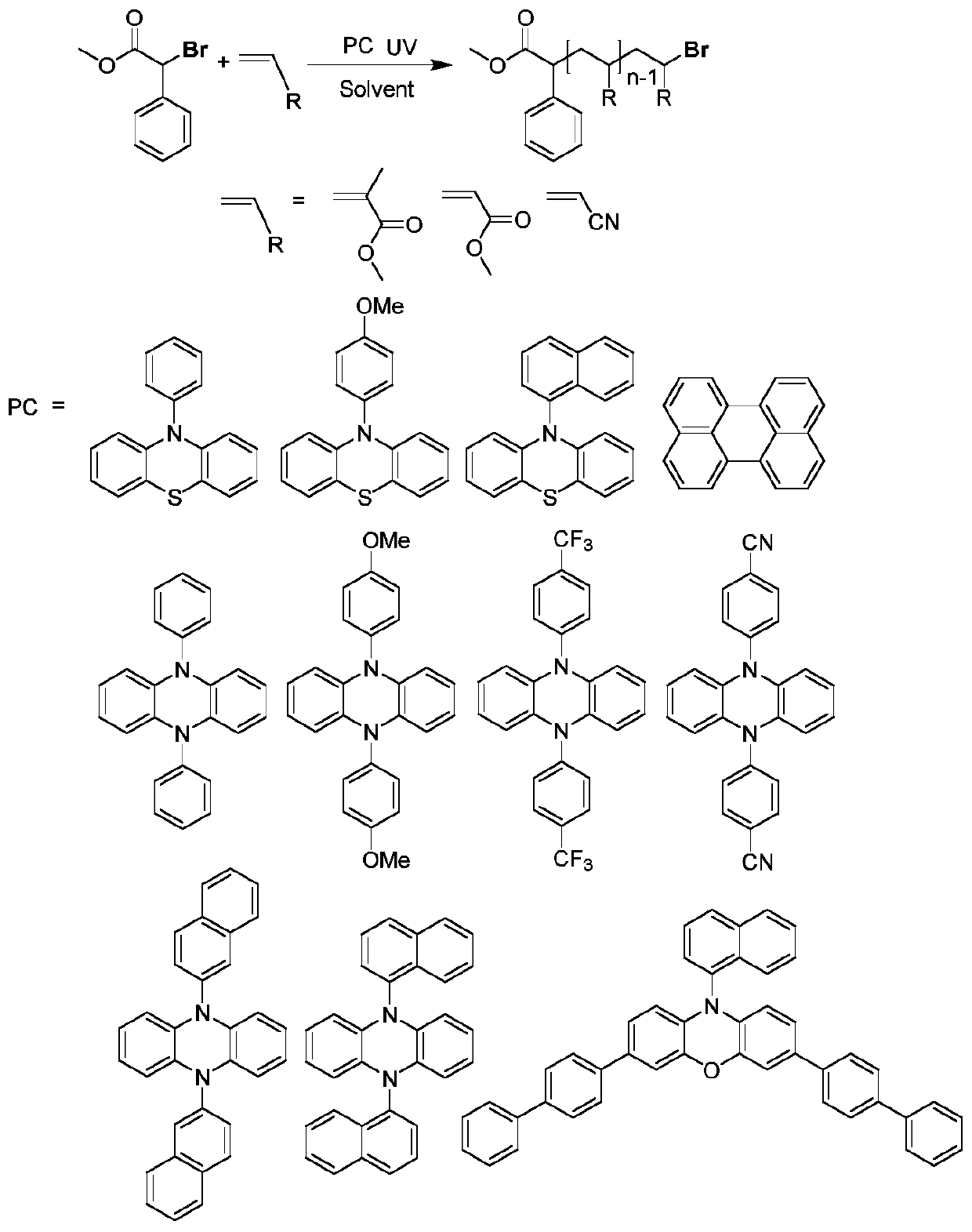
A method for photo-induced organocatalytic preparation of polymers at the microscale
A catalytic preparation and organic catalyst technology, applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical chemical processes, etc., can solve the problems of many side reactions, slow reaction speed, low initiator efficiency, etc., to achieve The effect of fast reaction speed, less side reactions, and uniform distribution of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
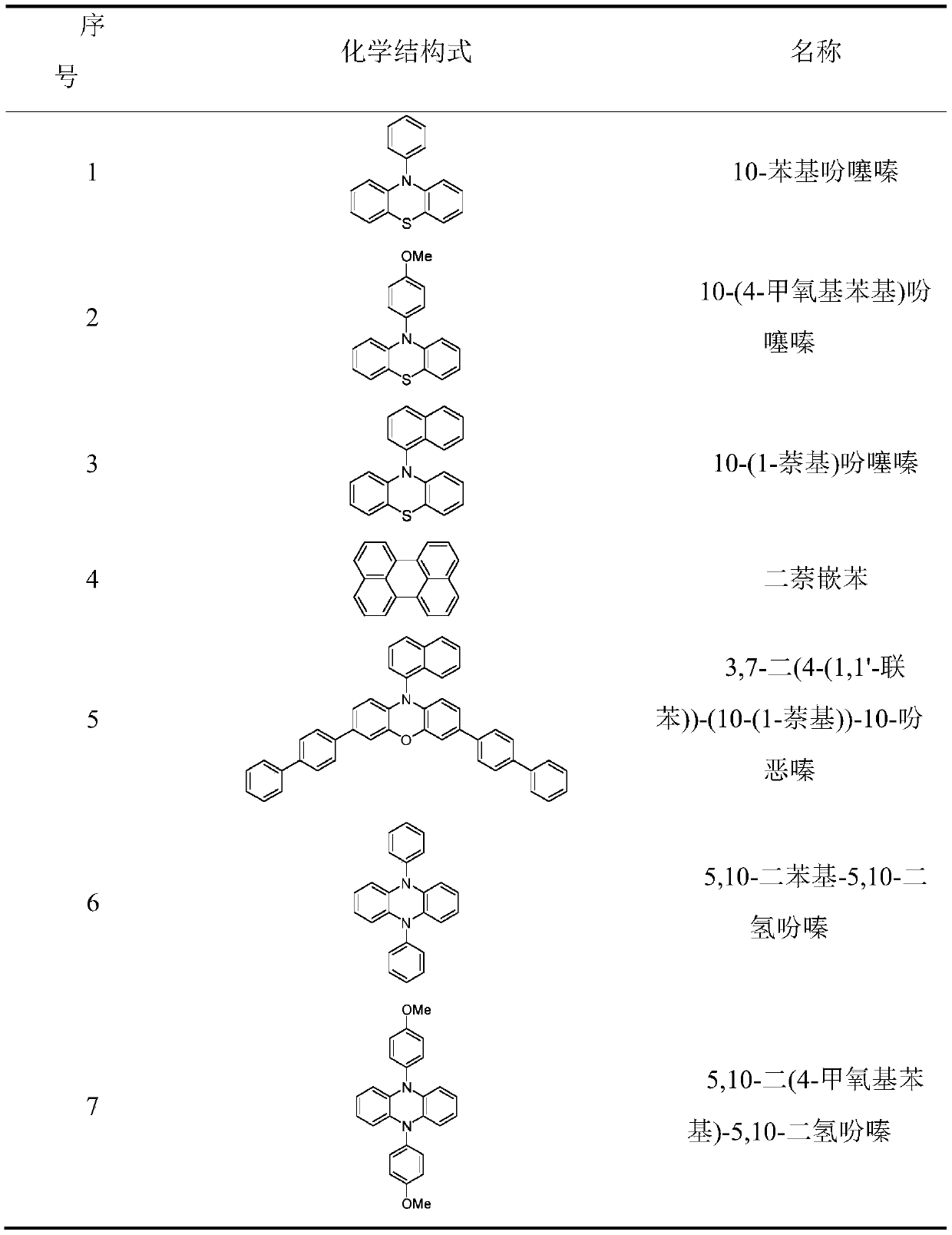
[0030] The preparation method of the organic catalyst refers to the following documents, 10-phenylphenothiazine reference [1], 10-(4-methoxyphenyl) phenothiazine reference [2], 10-(1-naphthalene base) phenothiazine reference [3], 3,7-bis(4-(1,1'-biphenyl))-(10-(1-naphthyl))-10-phenoxazine reference [4 ], 5,10-diphenyl-5,10-dihydrophenazine[5], 5,10-bis(4-methoxyphenyl)-5,10-dihydrophenazine[6], 5 ,10-bis(4-(trifluoromethyl)phenyl)-5,10-dihydrophenazine, 5,10-bis(4-(nitrile)phenyl)-5,10-dihydrophenazine or 5,10-bis(1-naphthyl)-5,10-dihydrophenazine reference [7], 5,10-bis(2-naphthyl)-5,10-dihydrophenazine reference [ 8]. The structures of the compounds prepared in the present invention are consistent with those reported in the literature. In addition, perylene can be purchased directly (CAS: 198-55-0, purity: 98%, manufacturer: Beijing Bailingwei Technology Co., Ltd.)
[0031] [1] Eric B, Kochi K. Journal of the chemical society-perkin transactions, 1995, 8:1057-1064.
[0...
Embodiment 1
[0040] At 20°C, the organic catalyst 10-phenylphenothiazine (31mg, 0.1128mmol) was dissolved in the solvent DMSO (30ml) under nitrogen protection to obtain a homogeneous solution for later use; the initiator α-bromophenylacetic acid methyl ester (345 μl, 2.256mmol) and monomeric MMA (12ml, 112.8mmol) are mixed uniformly and then pumped into the micro-mixer in the micro-reaction device simultaneously with the above-mentioned homogeneous solution respectively, control DMSO flow rate to be (0.059ml / min), MMA flow rate Be (0.024ml / min), pass in the microchannel reactor that has ultraviolet light (380nm) to irradiate after fully mixing, the residence time of mixed system in the microchannel reactor is 60min, and flow velocity is (0.083ml / min), fully Reaction, the product was collected and precipitated with a mixture of methanol and water (1:1). The product was washed 3 times with ethanol to remove residual monomer and solvent in the product. After the product was vacuum-dried at 3...
Embodiment 2
[0042] At 20°C, the organic catalyst 10-phenylphenothiazine (31mg, 0.1128mmol) was dissolved in the solvent DMSO (30ml) under the protection of nitrogen to obtain a homogeneous solution for later use; the initiator 2-bromopropionate methyl (250 μl, 2.256mmol) and monomeric MMA (12ml, 112.8mmol) are mixed uniformly and then pumped into the micro-mixer in the micro-reaction device simultaneously with the above-mentioned homogeneous solution respectively, control DMSO flow rate to be (0.119ml / min), MMA flow rate Be (0.048ml / min), pass in the microchannel reactor that has ultraviolet light (380nm) to irradiate after fully mixing, the residence time of mixed system in the microchannel reactor is 30min, and flow velocity is (0.167ml / min), fully Reaction, the product was collected and precipitated with a mixture of methanol and water (1:1). The product was washed 3 times with ethanol to remove residual monomer and solvent in the product. After the product was vacuum-dried at 30° C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com